Label: STERILE WATER- water injection, solution
- NDC Code(s): 0338-0013-06, 0338-0013-08, 0338-0013-29
- Packager: Baxter Healthcare Company
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated May 31, 2016
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
Sterile Water for Injection, USP is sterile, nonpyrogenic, distilled water in a Pharmacy Bulk Package. A Pharmacy Bulk Package is a container of a sterile preparation for parenteral use that contains many single doses. The contents are intended for use in a pharmacy admixture program and are restricted to the preparation of admixtures for intravenous infusion. No antimicrobial or other substance has been added. pH 5.5 (5.0 to 7.0). Osmolarity O mOsmol/L (calc.).
The VIAFLEX plastic container is fabricated from a specially formulated polyvinyl chloride (PL 146 Plastic). Exposure to temperatures above 25°C/77°F during transport and storage will lead to minor losses in moisture content. Higher temperatures lead to greater losses. It is unlikely that these minor losses will lead to clinically significant changes within the expiration period. The amount of water that can permeate from inside the container into the overwrap is insufficient to affect the solution significantly. Solutions in contact with the plastic container may leach out certain chemical components from the plastic in very small amounts; however, biological testing was supportive of the safety of the plastic container materials.
- CLINICAL PHARMACOLOGY
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
-
WARNINGS
This solution is for compounding only, not for direct infusion. Hemolysis may occur following infusion of Sterile Water for Injection, USP. Hemoglobin induced renal failure has been reported following hemolysis.
WARNING: This product contains aluminum that may be toxic. Aluminum may reach toxic levels with prolonged parenteral administration if kidney function is impaired. Premature neonates are particularly at risk because their kidneys are immature, and they require large amounts of calcium and phosphate solutions, which contain aluminum.
Research indicates that patients with impaired kidney function, including premature neonates, who receive parenteral levels of aluminum at greater than 4 to 5 µg/kg/day accumulate aluminum at levels associated with central nervous system and bone toxicity. Tissue loading may occur at even lower rates of administration.
- PRECAUTIONS
-
ADVERSE REACTIONS
The administration of a suitable admixture of prescribed drugs may be associated with adverse reactions because of the solution or the technique of administration including febrile response, infection at the site of injection, venous thrombosis or phlebitis extending from the site of injection, extravasation and hypervolemia. If an adverse reaction does occur, discontinue the infusion, evaluate the patient, institute appropriate therapeutic countermeasures and save the remainder of the fluid for examination if deemed necessary.
-
DOSAGE AND ADMINISTRATION
Following suitable admixture of prescribed drugs, the dosage is usually dependent upon the age, weight and clinical condition of the patient as well as laboratory determinations. See directions accompanying drugs.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit. Use of a final filter is recommended during administration of all parenteral solutions where possible.
Sterile Water for Injection, USP in the Pharmacy Bulk Package is intended for use in the preparation of sterile, intravenous admixtures. Additives may be incompatible with the fluid withdrawn from this container. Complete information is not available. Those additives known to be incompatible should not be used. Consult with pharmacist, if available. When compounding admixtures, use aseptic technique. Mix thoroughly. Do not store any unused portion of Sterile Water for Injection, USP.
DIRECTIONS FOR USE OF VIAFLEX PLASTIC PHARMACY BULK PACKAGE CONTAINER
To Open
Tear overwrap down side at slit and remove solution container. Visually inspect the container. If the outlet port protector is damaged, detached, or not present, discard container as solution path sterility may be impaired. Some opacity of the plastic due to moisture absorption during the sterilization process may be observed. This is normal and does not affect the solution quality or safety. The opacity will diminish gradually. Check for minute leaks by squeezing inner bag firmly, if leaks are found, discard solution as sterility may be impaired.
For compounding only, not for direct infusion.
Preparation for Admixing
- 1.
- The Pharmacy Bulk Package is to be used only in a suitable work area such as a laminar flow hood (or an equivalent clean air compounding area).
- 2.
- Suspend container from eyelet support.
- 3.
- Remove plastic protector from outlet port at bottom of container.
- 4.
- Attach solution transfer set. Refer to complete directions accompanying set.
Note: The closure shall be penetrated only one time with a suitable sterile transfer device or dispensing set which allows measured dispensing of the contents. - 5.
- VIAFLEX containers should not be written on directly since ink migration has not been investigated. Affix accompanying label for date and time of entry.
- 6.
- Once container closure has been penetrated, withdrawal of contents should be completed without delay. After initial entry, maintain contents at room temperature (25°C/77°F) and dispense within 4 hours.
-
HOW SUPPLIED
Sterile Water for Injection, USP is supplied in a VIAFLEX plastic Pharmacy Bulk Package container as follows:
2000 mL
2B0306
NDC 0338-0013-06
3000 mL
2B0307
NDC 0338-0013-08
5000 mL
2B0309
NDC 0338-0013-29
Exposure of pharmaceutical products to heat should be minimized. Avoid excessive heat. It is recommended the product be stored at room temperature (25°C/77°F).
Baxter Healthcare Corporation
Deerfield, IL 60015 USAPrinted in USA
Baxter, Viaflex, and PL 146 are trademarks of Baxter International Inc.
07 19 73 676
Rev. September 2014
Distributed in Canada by
Baxter Corporation
Mississauga, ON L5N 0C2 -
PACKAGE LABEL - PRINCIPLE DISPLAY PANEL
Container Label
LOT EXP
2B0306 2000 mL
NDC 0338-0013-06 DIN 02014882Sterile Water
For Injection USPPharmacy Bulk Package
Not For Direct InfusionRx Only
NO ANTIMICROBIAL OR OTHER SUBSTANCE HAS BEEN ADDED
pH 5.5 (5.0 TO 7.0) OSMOLARITY 0 mOsmol/L (CALC)
STERILE NONPYROGENICCONTAINS NO MORE THAN 25 μg/L OF ALUMINUM
ADDITIVES MAY BE INCOMPATIBLE WITH THE FLUID
WITHDRAWN FROM THIS CONTAINER CONSULT WITH
PHARMACIST IF AVAILABLE WHEN COMPOUNDING
ADMIXTURES USE ASEPTIC TECHNIQUE
MIX THOROUGHLY DO NOT STOREDOSAGE ADMIX FOR INTRAVENOUS ADMINISTRATION
AS DIRECTED BY A PHYSICIAN SEE ACCOMPANYING
DIRECTIONS FOR USE ONCE CONTAINER CLOSURE
HAS BEEN PENETRATED WITHDRAWAL OF
CONTENTS SHOULD BE COMPLETED WITHOUT
DELAY AFFIX ACCOMPANYING LABEL FOR DATE AND
TIME OF ENTRY DISPENSE CONTENTS WITHIN 4 HOURS
AFTER INITIAL ENTRYCAUTIONS SQUEEZE AND INSPECT INNER BAG WHICH
MAINTAINS PRODUCT STERILITY DISCARD IF LEAKS
ARE FOUND DO NOT USE UNLESS SOLUTION IS CLEAR
AND SEAL IS INTACTSTORE UNIT IN MOISTURE BARRIER OVERWRAP AT
ROOM TEMPERATURE (25°C/77°F) UNTIL READY TO USE
AVOID EXCESSIVE HEAT SEE INSERTVIAFLEX CONTAINER PL 146 PLASTIC
Baxter logo
BAXTER HEALTHCARE CORPORATION
DEERFIELD IL 60015 USAMADE IN USA
DISTRIBUTED IN CANADA BY
BAXTER CORPORATION
MISSISSAUGA ON L5N 0C2BAXTER PL 146 AND VIAFLEX ARE TRADEMARKS OF BAXTER INTERNATIONAL INC
1800
1600
1400
1200
1000
800
600
400
200
07-25-69-275/
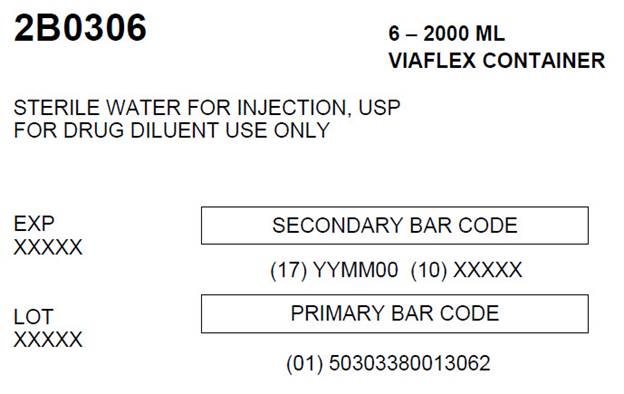
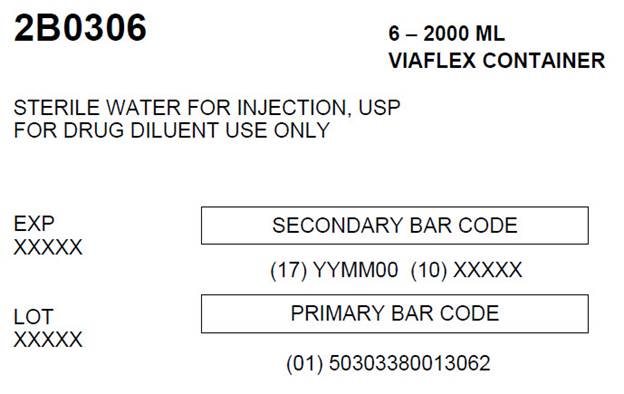
07-25-34-056Carton Label
2B0306 6 - 2000 ML
VIAFLEX CONTAINERSTERILE WATER FOR INJECTION, USP
FOR DRUG DILUENT USE ONLYEXP
XXXXXSECONDARY BAR CODE
(17) YYMM00 (10) XXXXX
LOT
XXXXXPRIMARY BAR CODE
(01) 50303380013062
-
INGREDIENTS AND APPEARANCE
STERILE WATER
water injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0338-0013 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength WATER (UNII: 059QF0KO0R) (WATER - UNII:059QF0KO0R) WATER 100 mL in 100 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0338-0013-06 2000 mL in 1 BAG; Type 0: Not a Combination Product 06/30/1982 2 NDC:0338-0013-08 3000 mL in 1 BAG; Type 0: Not a Combination Product 06/30/1982 3 NDC:0338-0013-29 5000 mL in 1 BAG; Type 0: Not a Combination Product 06/30/1982 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA018632 06/30/1982 Labeler - Baxter Healthcare Company (005083209) Establishment Name Address ID/FEI Business Operations Baxter Healthcare Corporation 059140764 ANALYSIS(0338-0013) , LABEL(0338-0013) , MANUFACTURE(0338-0013) , PACK(0338-0013) , STERILIZE(0338-0013) Establishment Name Address ID/FEI Business Operations Baxter Healthcare Corporation 194684502 ANALYSIS(0338-0013)