Label: BYNFEZIA PEN- octreotide acetate injection
-
Contains inactivated NDC Code(s)
NDC Code(s): 62756-452-36, 62756-452-37 - Packager: Sun Pharmaceutical Industries, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated February 19, 2020
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use BYNFEZIA PEN safely and effectively. See full prescribing information for BYNFEZIA PEN.
BYNFEZIA PEN (octreotide acetate) injection, for subcutaneous use
Initial U.S. Approval: 1988
INDICATIONS AND USAGE
BYNFEZIA Pen is a somatostatin analogue indicated for:
- Reduction of growth hormone (GH) and insulin-like growth factor 1 (IGF-1) [somatomedin C] in adult patients with acromegaly who have had inadequate response to or cannot be treated with surgical resection, pituitary irradiation, and bromocriptine mesylate at maximally tolerated doses (1.1)
- Treatment of severe diarrhea/flushing episodes associated with metastatic carcinoid tumors in adult patients (1.2)
- Treatment of profuse watery diarrhea associated with vasoactive intestinal peptide tumors (VIPomas) in adult patients (1.3)
Limitations of Use:
- In patients with acromegaly, the effect of BYNFEZIA Pen on improvement in clinical signs and symptoms, reduction in tumor size and rate of growth, has not been determined. (1.4)
- In patients with carcinoid syndrome and VIPomas, the effect of BYNFEZIA Pen on size, rate of growth and development of metastases, has not been determined. (1.4)
DOSAGE AND ADMINISTRATION
- Acromegaly: Initiate dosage at 50 mcg three times daily. Typical dosage is 100 mcg three times a day. (2.2)
- Carcinoid Tumors: 100-600 mcg daily in 2-4 divided doses for first 2 weeks (2.3)
- VIPomas: 200-300 mcg daily in 2-4 divided doses for first 2 weeks (2.4)
- See Full Prescribing Information for laboratory monitoring recommendations (2.1,2.2, 2.3, 2.4)
- Administered subcutaneously; alternate injection site periodically (2.5)
DOSAGE FORMS AND STRENGTHS
Injection: 2,500 mcg/mL octreotide as a 2.8 mL single-patient-use pen (3)
CONTRAINDICATIONS
Hypersensitivity to octreotide or any of the components of BYNFEZIA Pen (4)
WARNINGS AND PRECAUTIONS
- Cholelithiasis and Complications of Cholelithiasis: Monitor periodically. Discontinue if complications of cholelithiasis are suspected (5.1)
- Hypoglycemia or Hyperglycemia: Monitor glucose and adjust antidiabetic treatment as needed. (5.2)
- Thyroid Function Abnormalities: Hypothyroidism may occur. Assess baseline thyroid function before initiation and monitor periodically (5.3)
- Cardiac Function: Bradycardia, arrhythmia, or conduction abnormalities may occur. Drugs that have bradycardia effects may need dosage adjustments (5.4)
ADVERSE REACTIONS
Adverse reactions with BYNFEZIA Pen use include: (6.1)
- Diarrhea, loose stools, nausea and abdominal discomfort in > 30% of patients with acromegaly and > 5% of patients with carcinoid tumors and VIPomas
- Gallstones and sinus bradycardia in > 25% in patients with acromegaly
To report SUSPECTED ADVERSE REACTIONS, contact Sun Pharmaceutical Industries, Inc. at 1-800-818-4555 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
DRUG INTERACTIONS
Cyclosporine: May decrease cyclosporine concentrations with concomitant use of BYNFEZIA Pen (7.1)
Bromocriptine: May increase bromocriptine concentrations with concomitant use of BYNFEZIA Pen (7.3)
Other Concomitant Drugs: Dosage adjustment of calcium channel blockers and drugs that control fluid and electrolyte balance may be required (7.4)
USE IN SPECIFIC POPULATIONS
Females and Males of Reproductive Potential: Advise premenopausal females of the potential for an unintended pregnancy (8.3)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 4/2020
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Acromegaly
1.2 Carcinoid Tumors
1.3 Vasoactive Intestinal Peptide Tumors (VIPomas)
1.4 Limitations of Use
2 DOSAGE AND ADMINISTRATION
2.1 Laboratory Testing Prior to BYNFEZIA Pen Initiation
2.2 Recommended Dosage in Acromegaly
2.3 Recommended Dosage for Carcinoid Tumors
2.4 Recommended Dosage for VIPomas
2.5 Important Administration Instructions
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Cholelithiasis and Complications of Cholelithiasis
5.2 Hyperglycemia and Hypoglycemia
5.3 Thyroid Function Abnormalities
5.4 Cardiac Function Abnormalities
5.5 Decreased Vitamin B12 Levels and Abnormal Schilling's Tests
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Cyclosporine
7.2 Insulin and Oral Hypoglycemic Drugs
7.3 Bromocriptine
7.4 Other Concomitant Drug Therapy
7.5 Drug Metabolism Interactions
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment Of Fertility
13.2 Animal Toxicology and/or Pharmacology
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
1.1 Acromegaly
BYNFEZIA Pen is indicated to reduce blood levels of growth hormone (GH) and insulin-like growth factor 1 (IGF-1) [somatomedin C] in adult patients with acromegaly who have had inadequate response to or cannot be treated with surgical resection, pituitary irradiation, and bromocriptine mesylate at maximally tolerated doses.
1.2 Carcinoid Tumors
BYNFEZIA Pen is indicated for the treatment of adult patients with severe diarrhea and flushing episodes associated with metastatic carcinoid tumors.
1.3 Vasoactive Intestinal Peptide Tumors (VIPomas)
BYNFEZIA Pen is indicated for the treatment of adult patients with the profuse watery diarrhea associated with VIP-secreting tumors.
1.4 Limitations of Use
In patients with acromegaly, the effect of BYNFEZIA Pen on improvement in clinical signs and symptoms, reduction in tumor size, and rate of growth has not been determined.
In patients with carcinoid syndrome and VIPomas, the effect of BYNFEZIA Pen on the tumor size, rate of growth and development of metastases has not been determined.
-
2 DOSAGE AND ADMINISTRATION
2.1 Laboratory Testing Prior to BYNFEZIA Pen Initiation
- Assess baseline thyroid function before initiation of BYNFEZIA Pen therapy and monitor periodically [see Warnings and Precautions (5.3)].
2.2 Recommended Dosage in Acromegaly
- Initiate dosing at 50 mcg subcutaneously three times a day to improve tolerability to gastrointestinal adverse reactions [see Adverse Reactions (6.1)].
- Measure IGF-I levels every 2 weeks after BYNFEZIA Pen initiation or dosage change to guide titration. Alternatively, measuring growth hormone levels at 1-4 hour intervals for 8-12 hours after BYNFEZIA Pen administration can guide titration of dose. The goal is to achieve growth hormone levels less than 5 ng/mL or IGF-I levels within normal reference ranges for age and sex.
- The typical dosage is 100 mcg subcutaneously three times a day, but some patients require up to 500 mcg three times a day. Doses greater than 300 mcg daily seldom result in additional benefit; if an increase in dose fails to provide additional benefit, reduce the dosage. Once biochemical normalization, or maximal benefit is achieved, re-evaluate IGF-I or growth hormone levels at 6-month intervals.
- Withdraw BYNFEZIA Pen yearly for approximately 4 weeks from patients who have received irradiation to assess disease activity. If growth hormone or IGF-I levels increase and signs and symptoms recur, BYNFEZIA Pen therapy may be resumed at the dose used at the time of BYNFEZIA Pen discontinuation.
2.3 Recommended Dosage for Carcinoid Tumors
- The recommended daily dosage of BYNFEZIA Pen during the first 2 weeks of therapy ranges from 100 mcg daily to 600 mcg daily in 2 to 4 divided doses (mean daily dosage is 300 mcg).
- In clinical studies, the median daily maintenance dosage was approximately 450 mcg, but clinical and biochemical benefits were obtained in some patients with as little as 50 mcg, while others required doses up to 1,500 mcg daily. Experience with doses above 750 mcg daily is limited.
- Monitor patient’s urinary 5-hydroxyindole acetic acid (5-HIAA), plasma serotonin, and plasma Substance P.
2.4 Recommended Dosage for VIPomas
- The recommended daily dosage of BYNFEZIA Pen during the first 2 weeks of therapy ranges from 200 mcg daily to 300 mcg daily in 2 to 4 divided doses. Adjust the dosage to achieve a therapeutic response; daily dosage is 150 mcg to 750 mcg but usually doses above 450 mcg daily are not required.
- Monitor patient’s plasma vasoactive intestinal peptide (VIP).
2.5 Important Administration Instructions
- Inspect visually for particulate matter and discoloration. Only use BYNFEZIA Pen if the solution appears colorless with no visible particles.
- BYNFEZIA Pen should be at room temperature before injecting to reduce potential injection site reactions.
- Administer BYNFEZIA Pen by subcutaneous injection into the abdomen, the front of the middle thighs, or the back/outer area of the upper arms.
- Rotate injection sites so that the same site is not used repeatedly. Injection sites should be at least 2 inches away from your last injection site.
- Provide proper training to patients and/or caregivers on the administration of BYNFEZIA Pen prior to use according to the “Instructions for Use”.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Cholelithiasis and Complications of Cholelithiasis
BYNFEZIA Pen may inhibit gallbladder contractility and decrease bile secretion, which may lead to gallbladder abnormalities or sludge. There have been postmarketing reports of cholelithiasis (gallstones) resulting in complications, including cholecystitis, cholangitis, pancreatitis and requiring cholecystectomy in patients taking octreotide [see Adverse Reactions (6)]. Patients should be monitored periodically. If complications of cholelithiasis are suspected, discontinue BYNFEZIA Pen and treat appropriately.
5.2 Hyperglycemia and Hypoglycemia
BYNFEZIA Pen alters the balance between the counter-regulatory hormones, insulin, glucagon and growth hormone, which may result in hypoglycemia, hyperglycemia, or overt diabetes mellitus. In patients with acromegaly, 3% developed hypoglycemia and 16% developed hyperglycemia during treatment with octreotide. Severe hyperglycemia, subsequent pneumonia, and death following initiation of octreotide was reported in one patient with no history of hyperglycemia [see Adverse Reactions (6)].
In patients with type 1 diabetes mellitus, BYNFEZIA Pen is likely to affect glucose regulation, and insulin requirements may be reduced. Symptomatic hypoglycemia, sometimes severe, has been reported in these patients. In patients without diabetes and patients with type 2 diabetes with partially intact insulin reserves, BYNFEZIA Pen may result in decreases in plasma insulin levels and hyperglycemia. Monitor glycemic control for all patients and adjust antidiabetic treatment as necessary.
5.3 Thyroid Function Abnormalities
BYNFEZIA Pen suppresses secretion of thyroid stimulating hormone, which may result in hypothyroidism. In patients with acromegaly, 12% developed biochemical hypothyroidism, 8% developed goiter, and 4% required initiation of thyroid replacement therapy while receiving octreotide. Assess thyroid function at baseline and periodically during treatment with BYNFEZIA Pen [see Adverse Reactions (6)].
5.4 Cardiac Function Abnormalities
Cardiac conduction abnormalities have occurred during treatment with octreotide. In patients with acromegaly, bradycardia (<50 bpm) developed in 25%, conduction abnormalities occurred in 10% and arrhythmias occurred in 9% of patients during treatment with octreotide. Other ECG changes observed included QT prolongation, axis shifts, early repolarization, low voltage, R/S transition, and early R wave progression. These ECG changes may occur in patients with acromegaly. In one patient with acromegaly and severe congestive heart failure (CHF), initiation of octreotide resulted in worsening of CHF with improvement when drug was discontinued and worsened when octreotide was restarted [see Adverse Reactions (6)]. Dosage adjustments of concomitantly used drugs that have bradycardia effects (i.e. beta-blockers) may be necessary [see Drug Interactions (7.4)].
5.5 Decreased Vitamin B12 Levels and Abnormal Schilling's Tests
BYNFEZIA Pen may alter absorption of dietary fats in some patients. Decreased vitamin B12 levels and abnormal Schilling’s tests have been observed in some patients receiving octreotide. Monitor vitamin B12 levels during treatment with BYNFEZIA Pen.
-
6 ADVERSE REACTIONS
The following important adverse reactions are described below and elsewhere in the labeling:
- Cholelithiasis and Complications of Cholelithiasis [see Warnings and Precautions (5.1)]
- Hyperglycemia and Hypoglycemia [see Warnings and Precautions (5.2)]
- Thyroid Function Abnormalities [see Warnings and Precautions (5.3)]
- Cardiac Function Abnormalities [see Warnings and Precautions (5.4)]
- Decreased Vitamin B12 Levels and Abnormal Schilling’s Tests [see Warnings and Precautions (5.5)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trial of another drug and may not reflect the rates observed in practice.
The safety of BYNFEZIA Pen has been established based on clinical studies of octreotide acetate injection. Below is a description of the adverse reactions from the clinical studies.
Gallbladder AbnormalitiesGallbladder abnormalities, especially stones and/or biliary sludge, frequently develop in patients on chronic octreotide therapy. Single doses of octreotide have been shown to inhibit gallbladder contractility and decrease bile secretion in normal volunteers. In clinical trials [primarily patients with acromegaly or psoriasis (BYNFEZIA Pen is not indicated for the treatment of psoriasis)], the incidence of biliary tract abnormalities was 63% (27% gallstones, 24% sludge without stones, 12% biliary duct dilatation). The incidence of stones or sludge in patients who received octreotide for 12 months or longer was 52%. Less than 2% of patients treated with octreotide for 1 month or less developed gallstones. The incidence of gallstones did not appear related to age, sex or dose. Like patients without gallbladder abnormalities, the majority of patients developing gallbladder abnormalities on ultrasound had gastrointestinal symptoms. The symptoms were not specific for gallbladder disease. A few patients developed acute cholecystitis, ascending cholangitis, biliary obstruction, cholestatic hepatitis, or pancreatitis during octreotide therapy or following its withdrawal. One patient developed ascending cholangitis during octreotide therapy and died.
Cardiac
In acromegalics, sinus bradycardia (<50 bpm) developed in 25%; conduction abnormalities occurred in 10% and arrhythmias developed in 9% of patients.
GastrointestinalDiarrhea, loose stools, nausea and abdominal discomfort were each seen in 34%-61% of acromegalic patients in U.S. studies although only 2.6% of the patients discontinued therapy due to these symptoms. These symptoms were seen in 5%-10% of patients with other disorders.
The frequency of these symptoms was not dose-related, but diarrhea and abdominal discomfort generally resolved more quickly in patients treated with 300 mcg/day than in those treated with 750 mcg/day. Vomiting, flatulence, abnormal stools, abdominal distention, and constipation were each seen in less than 10% of patients.
In rare instances, gastrointestinal side effects may resemble acute intestinal obstruction, with progressive abdominal distension, severe epigastric pain, abdominal tenderness and guarding.
Hypoglycemia and HyperglycemiaHypoglycemia and hyperglycemia occurred in 3% and 16% of acromegalic patients, respectively, and in about 1.5% of other patients. Symptoms of hypoglycemia were noted in approximately 2% of patients.
HypothyroidismIn acromegalics, biochemical hypothyroidism alone occurred in 12% while goiter occurred in 6% during octreotide therapy. In patients without acromegaly, hypothyroidism has been reported in several patients.
Other Adverse ReactionsPain on injection was reported in 7.7%, headache in 6% and dizziness in 5%. Several cases of pancreatitis have been reported.
Other Adverse Reactions 1%-4%Other reactions observed in 1%-4% of patients, included fatigue, weakness, pruritus, joint pain, backache, urinary tract infection, cold symptoms, flu symptoms, injection site hematoma, bruise, edema, flushing, blurred vision, pollakiuria, fat malabsorption, hair loss, visual disturbance and depression.
Other Adverse Reactions <1%Reactions reported in less than 1% of patients are:
Gastrointestinal: hepatitis, jaundice, increase in liver enzymes, GI bleeding, hemorrhoids, appendicitis, gastric/peptic ulcer, gallbladder polyp;
Integumentary: rash, cellulitis, petechiae, urticaria, basal cell carcinoma;
Musculoskeletal: arthritis, joint effusion, muscle pain, Raynaud’s phenomenon;
Cardiovascular: chest pain, shortness of breath, thrombophlebitis, ischemia, congestive heart failure, hypertension, hypertensive reaction, palpitations, orthostatic BP decrease, tachycardia;
CNS: anxiety, libido decrease, syncope, tremor, seizure, vertigo, Bell’s Palsy, paranoia, pituitary apoplexy, increased intraocular pressure, amnesia, hearing loss, neuritis;
Respiratory: pneumonia, pulmonary nodule, status asthmaticus;
Endocrine: galactorrhea, hypoadrenalism, diabetes insipidus, gynecomastia, amenorrhea, polymenorrhea, oligomenorrhea, vaginitis;
Urogenital: nephrolithiasis, hematuria;
Hematologic: anemia, iron deficiency, epistaxis;
Miscellaneous: otitis, allergic reaction, increased CK, weight loss.
Antibodies to OctreotideEvaluation of 20 patients treated for at least 6 months has failed to demonstrate titers of antibodies exceeding background levels. However, antibody titers to octreotide were subsequently reported in three patients and resulted in prolonged duration of drug action in two patients. Anaphylactoid reactions, including anaphylactic shock, have been reported in several patients receiving octreotide.
6.2 Postmarketing Experience
The following adverse reactions have been identified during the postapproval use of octreotide acetate injection. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Gastrointestinal:intestinal obstruction
Hematologic:thrombocytopenia
-
7 DRUG INTERACTIONS
7.1 Cyclosporine
Concomitant administration of BYNFEZIA Pen with cyclosporine may decrease blood levels of cyclosporine and result in transplant rejection.
7.2 Insulin and Oral Hypoglycemic Drugs
Octreotide inhibits the secretion of insulin and glucagon. Monitor blood glucose levels upon BYNFEZIA Pen initiation or dose adjustment. Patients with diabetes mellitus may require dose adjustments of insulin or other antidiabetic agents [see Warnings and Precautions (5.2)].
7.3 Bromocriptine
Concomitant administration of BYNFEZIA Pen and bromocriptine increases the availability of bromocriptine.
7.4 Other Concomitant Drug Therapy
Patients receiving beta blockers, calcium channel blockers, or agents to control fluid and electrolyte balance, may require dose adjustments of these therapeutic agents.
Octreotide has been associated with alterations in nutrient absorption, so it may have an effect on absorption of orally administered drugs.
7.5 Drug Metabolism Interactions
Limited published data indicate that somatostatin analogs might decrease the metabolic clearance of compounds known to be metabolized by cytochrome P450 enzymes, which may be due to the suppression of growth hormones. Since octreotide may have this effect, concomitant use of BYNFEZIA Pen with other drugs mainly metabolized by CYP3A4 and which have a low therapeutic index (e.g., quinidine) should be used with caution and increased monitoring may be required.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Available data from case reports with octreotide acetate use in pregnant women are insufficient to identify a drug-associated risk of major birth defects, miscarriage or adverse maternal or fetal outcomes. In animal reproduction studies, no adverse developmental effects were observed with intravenous administration of octreotide to pregnant rats and rabbits during organogenesis at doses 7 and 13-times, respectively, the maximum recommended human dose (MRHD) of 1,500 mcg/day based on body surface area. Transient growth retardation, with no impact on postnatal development, was observed in rat offspring from a pre- and post-natal study of octreotide at intravenous doses below the MRHD based on body surface area (see Data).
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2%-4% and 15%-20%, respectively.
Data
Animal Data
In embryo-fetal development studies in rats and rabbits, pregnant animals received intravenous doses of octreotide up to 1 mg/kg/day during the period of organogenesis. A slight reduction in body weight gain was noted in pregnant rats at 0.1 and 1 mg/kg/day. There were no maternal effects in rabbits or embryo-fetal effects in either species up to the maximum dose tested. At 1 mg/kg/day in rats and rabbits, the dose multiple was approximately 7 and 13 times, respectively, at the highest recommended human dose of 1,500 mcg/day based on body surface area.
In a pre- and post-natal development rat study at intravenous doses of 0.02–1 mg/kg/day, a transient growth retardation of the offspring was observed at all doses which was possibly a consequence of growth hormone inhibition by octreotide. The doses attributed to the delayed growth are below the human dose of 1,500 mcg/day, based on body surface area.
8.2 Lactation
Risk Summary
There is no information available on the presence of octreotide in human milk, the effects of the drug on the breastfed infant, or the effects of the drug on milk production. Studies show that octreotide administered subcutaneously passes into the milk of lactating rats (see Data). When a drug is present in animal milk, it is likely that the drug will be present in human milk. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for BYNFEZIA Pen, and any potential adverse effects on the breastfed child from BYNFEZIA Pen or from the underlying maternal condition.
Data
Following a subcutaneous dose (1 mg/kg) of octreotide to lactating rats, transfer of octreotide into milk was observed at a low concentration compared to plasma (milk/plasma ratio of 0.009).
8.3 Females and Males of Reproductive Potential
Discuss the potential for unintended pregnancy with premenopausal women as the therapeutic benefits of a reduction in GH levels and normalization of IGF-1 concentration in acromegalic females treated with octreotide may lead to improved fertility.
8.4 Pediatric Use
Safety and efficacy of BYNFEZIA Pen in pediatric patients have not been established.
In post-marketing reports, serious adverse reactions, including hypoxia, necrotizing enterocolitis, and death, have been reported with octreotide injection use in pediatric patients, most notably in children under 2 years of age.8.5 Geriatric Use
Clinical studies of octreotide did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
-
10 OVERDOSAGE
A limited number of accidental overdoses of octreotide in adults have been reported. The doses ranged from 2,400 mcg/day to 6,000 mcg/day administered by continuous infusion or subcutaneously 1,500 mcg three times a day. Adverse reactions in some patients included arrhythmia, hypotension, cardiac arrest, brain hypoxia, pancreatitis, hepatitis steatosis, hepatomegaly, lactic acidosis, flushing, diarrhea, lethargy, weakness, and weight loss.
If overdose occurs, contact Poison Control (1-800-222-1222) for latest recommendations.
-
11 DESCRIPTION
BYNFEZIA Pen (octreotide acetate) injection is a sterile, clear, colorless solution of octreotide, acetate in buffered lactic acid solution. BYNFEZIA Pen is somatostatin analogue. Octreotide acetate is chemically known as L-Cysteinamide, D-phenylalanyl-L-cysteinyl-L-phenylalanyl-D-tryptophyl-L-lysyl-L-threonyl-N-[2-hydroxy-1-(hydroxymethyl)propyl]-, cyclic (2→7)-disulfide; [R-(R*, R*)] acetate salt. It is a long-acting octapeptide with pharmacologic actions mimicking those of the natural hormone somatostatin.
The molecular weight of octreotide acetate is 1019.3 (free peptide, C49H66N10O10S2) and its amino acid sequence is:
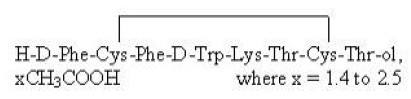
BYNFEZIA Pen (octreotide acetate) injection is available as disposable single-patient-use pen with a deliverable volume of 2.8 mL.
Each milliliter of BYNFEZIA Pen contains 2,500 mcg octreotide (present as octreotide acetate, USP), 3.4 mg lactic acid USP, 22.5 mg mannitol USP, 5 mg phenol USP, and Water for Injection USP. The pH of the solution is adjusted to 4.2 ± 0.3 by the addition of aqueous sodium bicarbonate solution.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
BYNFEZIA Pen (octreotide acetate) exerts pharmacologic actions similar to the natural hormone, somatostatin. It is an even more potent inhibitor of growth hormone, glucagon, and insulin than somatostatin. Like somatostatin, it also suppresses LH response to GnRH, decreases splanchnic blood flow, and inhibits release of serotonin, gastrin, vasoactive intestinal peptide, secretin, motilin, and pancreatic polypeptide.
By virtue of these pharmacological actions, octreotide has been used to treat the symptoms associated with metastatic carcinoid tumors (flushing and diarrhea), and VIP secreting adenomas (watery diarrhea).
12.2 Pharmacodynamics
Octreotide substantially reduces growth hormone and/or IGF-I levels in patients with acromegaly.
Single doses of octreotide have been shown to inhibit gallbladder contractility and to decrease bile secretion in normal volunteers. In clinical trials the incidence of gallstone or biliary sludge formation was markedly increased [see Warnings and Precautions (5.1)].
Octreotide may suppress secretion of thyroid stimulating hormone (TSH) [see Warnings and Precautions (5.3)].
12.3 Pharmacokinetics
Absorption
After subcutaneous injection, octreotide is absorbed and completely from the injection site. Peak concentrations of 5.2 ng/mL (100 mcg dose) were reached 0.4 hours after dosing. Peak concentrations and area under the curve values were dose proportional after subcutaneous single doses up to 500 mcg and after subcutaneous multiple doses up to 500 mcg three times a day (1,500 mcg/day).
Distribution
In healthy volunteers the distribution of octreotide from plasma was rapid (tα1/2 = 0.2 h), the volume of distribution (Vdss) was estimated to be 13.6 L, and the total body clearance ranged from 7 L/hr to 10 L/hr. In blood, the distribution into the erythrocytes was found to be negligible and about 65% was bound in the plasma in a concentration-independent manner. Binding was mainly to lipoprotein and, to a lesser extent, to albumin.
Elimination
The elimination of octreotide from plasma had an apparent half-life of 1.7 to 1.9 hours compared with 1-3 minutes with the natural hormone. The duration of action of octreotide injection is variable but extends up to 12 hours depending upon the type of tumor. About 32% of the dose is excreted unchanged into the urine.
In patients with acromegaly, the pharmacokinetics differ somewhat from those in healthy volunteers. A mean peak concentration of 2.8 ng/mL (100 mcg dose) was reached in 0.7 hours after subcutaneous dosing. The volume of distribution (Vdss) was estimated to be 21.6 ± 8.5 L and the total body clearance was increased to 18 L/hr. The mean percent of the drug bound was 41.2%. The disposition and elimination half-lives were similar to normals.
Specific Populations
Geriatric Patients
In an elderly population, dose adjustments may be necessary due to a significant increase in the half-life (46%) and a significant decrease in the clearance (26%) of the drug [see Use in Specific Populations (8.5)].
Patients with Renal Impairment
In patients with renal impairment the elimination of octreotide from plasma was prolonged and total body clearance reduced. In mild renal impairment (ClCR 40-60 mL/min) octreotide t1/2 was 2.4 hours and total body clearance was 8.8 L/hr, in moderate impairment (ClCR 10-39 mL/min) t1/2 was 3.0 hours and total body clearance 7.3 L/hr, and in severely renally impaired patients not requiring dialysis (ClCR <10 mL/min) t1/2 was 3.1 hours and total body clearance was 7.6 L/hr. In patients with severe renal failure requiring dialysis, total body clearance was reduced to about half that found in healthy subjects (from approximately 10 L/hr to 4.5 L/hr) [see Use in Specific Populations (8.6)].
Patients with Hepatic Impairment
Patients with liver cirrhosis showed prolonged elimination of drug, with octreotide t1/2 increasing to 3.7 hr and total body clearance decreasing to 5.9 L/hr, whereas patients with fatty liver disease showed t1/2 increased to 3.4 hr and total body clearance of 8.2 L/hr.
Drug Interaction Studies
Limited published data indicate that somatostatin analogs may decrease the metabolic clearance of compounds known to be metabolized by cytochrome P450 enzymes [see Drug Interactions (7.5)].
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment Of Fertility
Studies in laboratory animals have demonstrated no mutagenic potential of octreotide.
No carcinogenic potential was demonstrated in mice treated subcutaneously for 85-99 weeks at doses up to 2,000 mcg/kg/day (8 x the human exposure based on body surface area). In a 116-week subcutaneous study in rats, a 27% and 12% incidence of injection site sarcomas or squamous cell carcinomas was observed in males and females, respectively, at the highest dose level of 1,250 mcg/kg/day (10 x the human exposure based on body surface area) compared to an incidence of 8%-10% in the vehicle-control groups. The increased incidence of injection site tumors was most probably caused by irritation and the high sensitivity of the rat to repeated subcutaneous injections at the same site. Rotating injection sites would prevent chronic irritation in humans. There have been no reports of injection site tumors in patients treated with octreotide for up to 5 years. There was also a 15% incidence of uterine adenocarcinomas in the 1,250 mcg/kg/day females compared to 7% in the saline-control females and 0% in the vehicle-control females. The presence of endometritis coupled with the absence of corpora lutea, the reduction in mammary fibroadenomas, and the presence of uterine dilatation suggest that the uterine tumors were associated with estrogen dominance in the aged female rats which does not occur in humans.
Octreotide did not impair fertility in rats at doses up to 1,000 mcg/kg/day, which represents 7 x the human exposure based on body surface area. -
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
BYNFEZIA Pen (octreotide acetate) injection, 2,500 mcg/mL octreotide is a clear colorless solution and it is available as:
Dosage Unit
Package Size
NDC #
2.8 mL single-patient-use prefilled pen
Carton of 1
NDC 62756-452-36
2.8 mL single-patient-use prefilled pen
Carton of 2
NDC 62756-452-37
16.2 Storage and Handling
Store BYNFEZIA Pen in the refrigerator between 2° to 8° C (36° to 46° F) in the carton. Protect the pen from light. After first use store pens at controlled room temperature between 20°C to 25°C (68°F to 77°F). Excursions between 15°C (59°F) and 30°C (86°F) are allowed for up to 28 days. Discard the pen 28 days after first use.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Instructions for Use).
Cholelithiasis and Complications of CholelithiasisAdvise patients to contact their healthcare provider if they experience signs or symptoms of gallstones (cholelithiasis) or complications of cholelithiasis (e.g., cholecystitis, cholangitis and pancreatitis) [see Warnings and Precautions (5.1)].
Hypoglycemia and HyperglycemiaAdvise patients to contact their healthcare provider if they have problems with blood sugar levels, either too high (hyperglycemia) or too low (hypoglycaemia) [see Warnings and Precautions (5.2)].
Thyroid Function AbnormalitiesInform patients that their thyroid function will be assessed at baseline and periodically during treatment [see Warnings and Precautions (5.3)].
Cardiac Function AbnormalitiesInform patients to contact the health care provider in case they notice irregular heart beat [see Warnings and Precautions (5.4)].
Decreased Vitamin B12 Levels and Abnormal Schilling’s TestsInform patients that Vitamin B12 levels may be monitored during the treatment [see Warnings and Precautions (5.5)].
PregnancyInform female patients that treatment with BYNFEZIA Pen may result in unintended pregnancy [see Use in Specific Populations (8.3)].
Distributed by:Sun Pharmaceutical Industries, Inc.
Cranbury, NJ 08512
Manufactured by:Sun Pharmaceutical Industries Ltd.
Halol-Baroda Highway,
Halol-389 350, Gujarat, India
Revised: 04/2020
-
INSTRUCTIONS FOR USE
INSTRUCTIONS FOR USE
Bynfezia Pen (ben-FEZ-ee-uh PEN)
(octreotide acetate)
Prefilled Pen
Read these instructions before you start using the Bynfezia Pen. It is important that you understand and follow these instructions to use the pen correctly. Bynfezia Pen is a prefilled pen that you or your caregiver can use to give more than 1 dose of medicine.
Ask your healthcare provider about your dose of Bynfezia Pen and how to inject Bynfezia Pen the right way before you inject it for the first time. If your healthcare provider decides that you or your caregiver can give your Bynfezia Pen at home, you or your caregiver should receive training on how to use the pen.
Contact your healthcare provider if you or your caregiver have any questions.
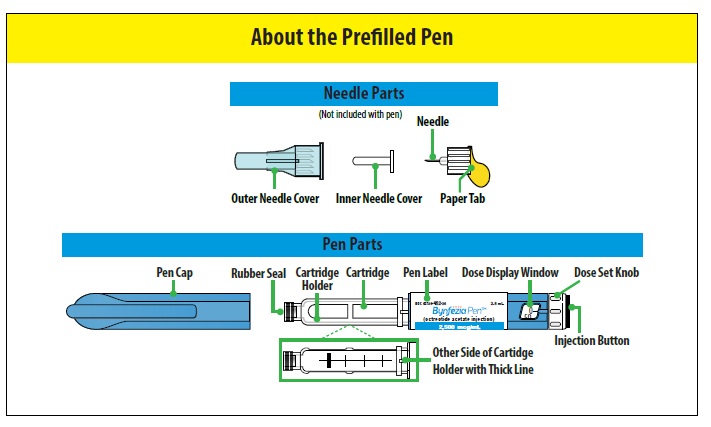
Important - Do not use a pen for more than 28 days after first use. After 28 days throw away (dispose of) the pen, even if the pen still has medicine in it.
- Do not place the pen in water or any other liquid.
- Keep your pen and needles out of the reach of children.
- Do not use if any part of your pen appears broken or damaged.
- If you drop your pen, prime it before you use it again to make sure the pen still works correctly (see step 3. Prime Your Pen).
- Do not take Bynfezia Pen solution out of the pen and put it into a syringe.
- Do not share your pen or needles with another person. You may give an infection to them or get an infection from them.
- Do not remove the outer needle cover or inner needle cover until you are ready to inject.
- Do not press the injection button unless a needle is attached to the pen.
How should I store my Bynfezia Pen? Before First Use:
- Store new unused pens in the refrigerator between 36°F to 46°F (2°C to 8°C) in the carton.
- Protect the pen from light.
- Do not freeze. Throw away (dispose of) the pen if it has been frozen.
- Do not store the pen in direct sunlight.
- Store the pen at room temperature between 68°F to 77°F (20°C to 25°C) for up to 28 days.
- Store the pen with the pen cap on.
- Throw away (dispose of) the pen 28 days after first use in a sharps container, even if the pen still has medicine in it (see step 6. Additional Disposal Information).
- Do not store a pen with a needle attached.
How do I give a dose larger than 200 mcg (more than 1 injection)? - Turn the dose set knob to 200 mcg (highest dose setting) and give the first injection. Choose a different injection site for each injection at least 2 inches from the area you used for your last injection.
- Calculate the remaining dose by subtracting 200 mcg from the prescribed dose.
- Turn the dose set knob to the line for your remaining dose up to the highest dose setting of 200 mcg. Give the second injection at least 2 inchesaway from the first injection.
- You may need more injections to give your total prescribed dose (see examples below).
Example Dose Steps to give a total dose larger than 200 mcg Number of injections needed to give the total dose For example, if your total dose is 300 mcg - Turn the dose set knob to 200 mcg for your first injection.
- Your remaining dose is 100 mcg.
- For the second injection, turn your dose set knob to 100 mcg to give the remaining dose.
2For example, if your total dose is 450 mcg - Turn the dose set knob to 200 mcg for your first injection.
- Your remaining dose is 250 mcg.
- Turn the dose set knob to 200 mcg for your second injection.
- Your remaining dose is 50 mcg.
- For the third injection, turn your dose set knob to 50 mcg to give the remaining dose.
31. Check the Pen
A. Wash your hands with soap and water (Figure A).
B. Check the expiration (EXP) date (Figure B).
- Check the pen label to make sure the expiration date has not passed.
Do not use if the expiration date has passed.
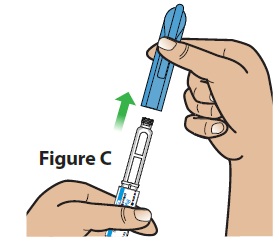
C. Pull the pen cap straight off the pen (Figure C).
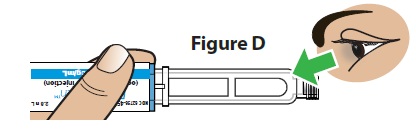
D. Check the medicine (Figure D).
- The medicine should be clear and colorless. You may see small air bubbles in the pen, which is normal and will not affect the dose.
Do not use if the medicine looks cloudy, colored, or has lumps or particles in it. The pen cartridge may look empty because the medicine is clear and colorless.
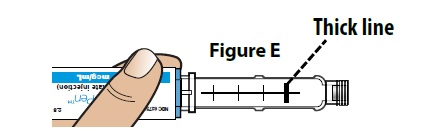
- Make sure you have enough medicine left in the pen to inject the full dose. When the plunger moves pass the thick line, your pen is almost empty (Figure E). If the dose set knob does not let you dial your prescribed dose, this means there is not enough medicine left in your pen. Throw away (dispose of) the pen and use a new pen for the injection.
E. Allow the pen to reach room temperature.
- If the pen was not stored at room temperature between 68°F to 77°F (20°C to 25°C), allow it to reach room temperature for 20 to 30 minutes before injecting. This will reduce the chance of getting a reaction at the injection site.
- See the section "How should I store my Bynfezia Pen?" for more information.
2. Attach a Needle
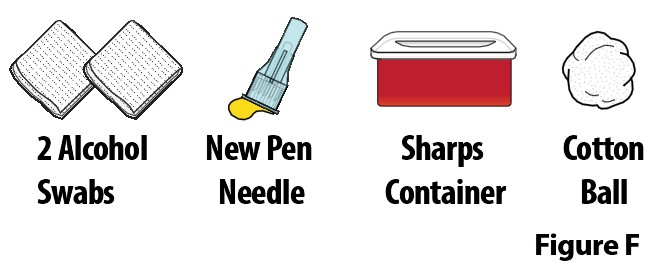
A. Gather the following additional supplies (Figure F):
- 2 alcohol swabs
- new pen needle
- sharps container
- cotton ball
B. Wipe the rubber seal on the pen with an alcohol swab (Figure G).
C. Attach a new needle.
- Use only 31 gauge, 5 mm length disposable pen needles. If you have questions about which needle to use, ask your healthcare provider.
- Peel off the paper tab from the needle (Figure H).
- Push the needle with cap straight down onto the pen and screw it on to the pen by turning to the right (clockwise) until the needle feels secure (Figure I).
D. Remove the outer needle cover and set it aside (Figure J).
- Save the outer needle cover for use when you remove the needle in step 6.
E. Remove the inner needle cover and throw it away (Figure K).
Are you using a new pen?
- If Yes, complete step 3.
- If No, skip step 3 and go to step 4.
3. Prime Your Pen
Note: The following steps are only needed if you are using a new pen for the first time or if you drop the pen.
To prime the pen, follow the steps below to dial the pen to 100 mcg and press the injection button until a stream of medicine comes from the needle tip.
- If you have already primed the pen, go to step 4 to prepare and give the injection.
- Do not prime the pen before each dose. This will waste medicine.
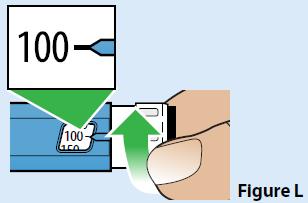
A. Turn the dose set knob to dial the pen to 100 mcg (Figure L).
B. Hold the pen with the needle pointing up.
C. Press the injection button all the way in until it stops and the dose display window returns to “0” (Figure M).
D. A stream of medicine should be seen coming from the needle tip (Figure N).
If you do not see a stream of medicine coming from the needle tip, repeat the priming steps.
If you still do not see a stream of medicine coming from the needle tip after you repeat the priming steps 3 times, the pen may be damaged. Throw away (dispose of) the pen and use a new pen. Call Sun Pharmaceutical Industries, Inc. at 1-800-818-4555or your healthcare provider for help or to get a new pen.
4. Prepare the Injection
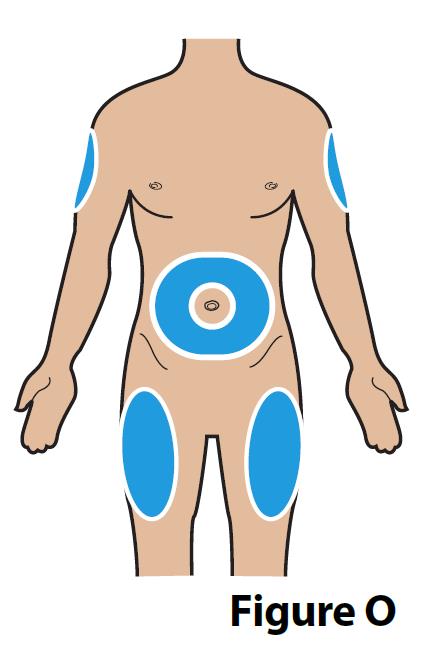
A. Choose an injection site (see Figure O) for injection under the skin (subcutaneous).
- When giving yourself the injection: Inject into the stomach at least 2 inches away from the belly button (navel) or inject into the front of the middle thighs (Figure O).
- When giving someone else the injection: you may also inject into the back outer area of the upper arms (Figure O).
- Always change (rotate) the injection site with each injection. Your injection site should be at least 2 inches away from your last injection site.
B. Clean your injection site with an alcohol swab (Figure P).
- Wipe the skin with an alcohol swab. Let the injection site dry before you inject your dose.
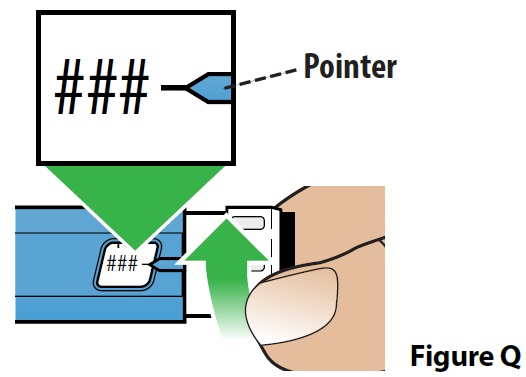
C. Dial your dose (Figure Q).
- Turn the dose set knob until you see the prescribed dose in the dose display window. The dose number and black line should line up with the pointer. The pen can be used to deliver 50 mcg, 100 mcg, 150 mcg and 200 mcg doses.
If your prescribed dose is more than 200 mcg, see the section “How should I give a dose larger than 200 mcg (more than 1 injection)?” in these instructions.
- It is normal to hear a “clicking” sound as you turn the dose set knob.
- If you accidentally dial past the prescribed dose, turn the dose set knob back down to the correct dose.
Note: The pen cannot be dialed past the number of micrograms (mcgs) of medicine left in the pen. If the pen does not have enough medicine to give the full dose, throw away (dispose of) the pen and use a new one.
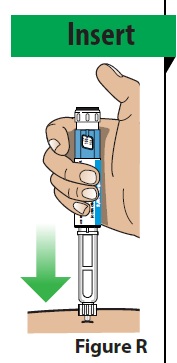
5. Give the Injection A. Insert the needle straight into the injection site (Figure R).
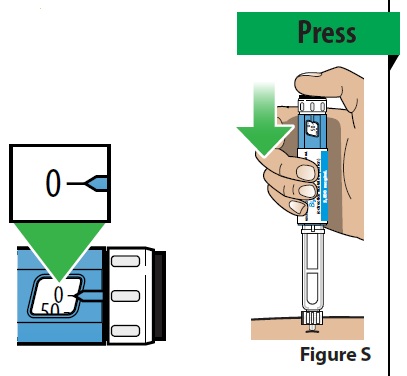
B. Deliver your dose.
- Deliver your dose by slowly pressing the injection button all the way down until it stops. The number in the dose display window will go back to “0” when the injection is complete (Figure S).
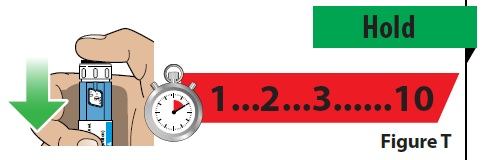
C. Continue to hold the injection button down and slowly count to 10 to make sure the full dose of medicine is given (Figure T).
D. Remove the pen from the injection site.
- Lift the pen straight up from the injection site (Figure U).
- If you see 1 or 2 drops of medicine on the needle tip, this is normal and will not affect your dose.
- If you see more than 2 drops of medicine on the needle tip, or you see liquid around the injection site after your injection, you may not have received your full dose.
Note: As the pen is used, a plunger will appear in the cartridge and move towards the thick line.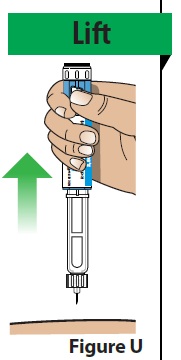
6. After the Injection
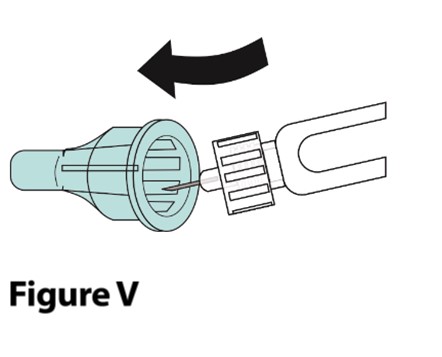
A. Carefully place the outer needle cover back onto the needle.
- Place the outer needle cover on a flat surface. Hold the syringe with the needle attached in 1 hand and carefully slip the needle into the outer needle cover without using the other hand. Push the outer needle cover completely on (Figure V).
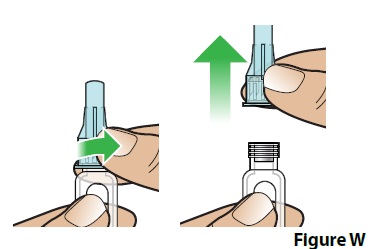
B. Unscrew and remove the covered needle (Figure W).
- Squeeze the lower part of the covered needle while turning it to the left (counter clockwise).
- Keep turning until the covered needle lifts away from the pen. It may take several turns for the covered needle to lift away from the pen.
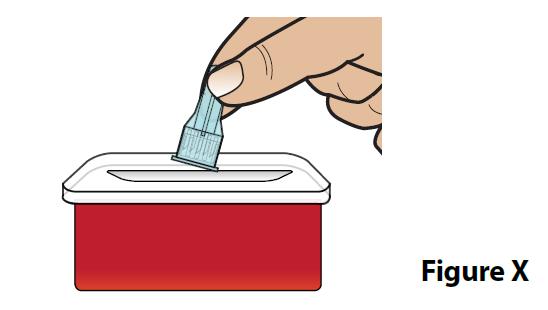
C. Throw away (dispose of) the covered needle in a FDA-cleared sharps container (Figure X).
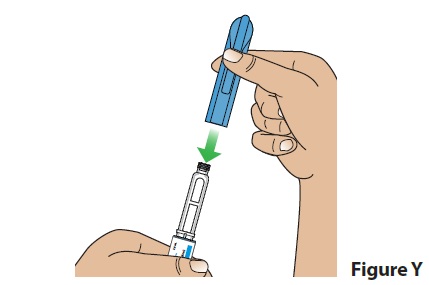
D. Put the pen cap back on the pen (Figure Y) and store the pen.
- After use, store pens with the cap on at room temperature between 68°F to 77°F (20°C to 25°C) or in the refrigerator between 36°F to 46°F (2°C to 8°C) for up to 28 days.
- See the section "How should I store my Bynfezia Pen?" for more information on storing your pen.
E. Treat the injection site.
- If needed, press the injection site lightly with a cotton ball or an alcohol swab. Do not rub the area.
Cleaning the Pen
- Wipe the outside of the pen with a clean, damp cloth.
- White particles may appear on the outside tip of the cartridge during normal use. You may remove them with an alcohol swab.
- Do not put the pen in water or any other liquid.
Additional Disposal Information
Put used pens and needles in a FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) pens and needles in the household trash.
If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- made of a heavy-duty plastic,
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- upright and stable during use,
- leak-resistant, and
- properly labeled to warn of hazardous waste inside the container.
When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA's website at: http://www.fda.gov/safesharpsdisposal
- Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this.
- Do not recycle your used sharps disposal container.
- Always keep the sharps disposal container out of the reach of children and pets.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Distributed by:
Sun Pharmaceutical Industries, Inc.
Cranbury, NJ 08512
Manufactured by:
Sun Pharmaceutical Industries Ltd.
Halol-Baroda Highway,
Halol-389 350, Gujarat, India
For more information, call 1-800-818-4555.
Issued: 04/2020
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
BYNFEZIA PEN
octreotide acetate injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:62756-452 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTREOTIDE ACETATE (UNII: 75R0U2568I) (OCTREOTIDE - UNII:RWM8CCW8GP) OCTREOTIDE 2.5 mg in 1 mL Inactive Ingredients Ingredient Name Strength LACTIC ACID (UNII: 33X04XA5AT) 3.4 mg in 1 mL MANNITOL (UNII: 3OWL53L36A) 22.5 mg in 1 mL PHENOL (UNII: 339NCG44TV) 5 mg in 1 mL SODIUM BICARBONATE (UNII: 8MDF5V39QO) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62756-452-37 2 in 1 CARTON 04/29/2020 1 2.8 mL in 1 CARTRIDGE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 2 NDC:62756-452-36 1 in 1 CARTON 04/29/2020 2 2.8 mL in 1 CARTRIDGE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA213224 04/29/2020 Labeler - Sun Pharmaceutical Industries, Inc. (146974886) Establishment Name Address ID/FEI Business Operations Sun Pharmaceutical Industries Limited 725959238 ANALYSIS(62756-452) , MANUFACTURE(62756-452)


