SWEATBLOCK- aluminum chloride solution
DC Alpine Partners, LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
SWEATBLOCK Clinical Strength Antiperspirant
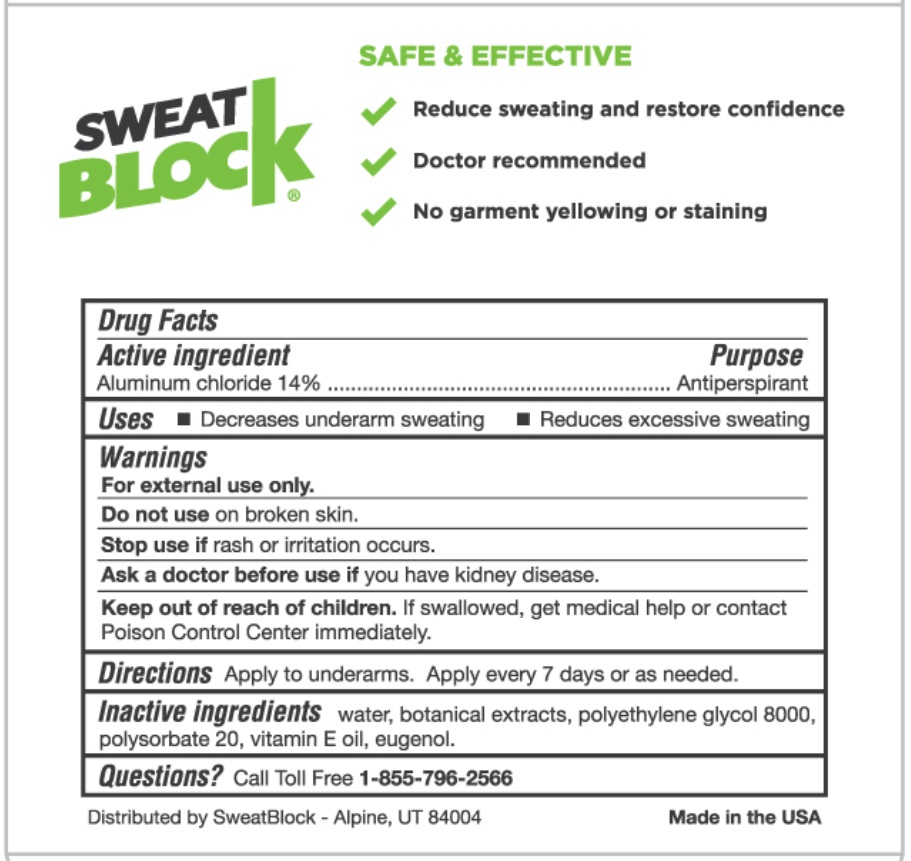
SWEATBLOCK
SAFE & EFFECTIVE
- Reduce sweating and restore confidence
- Doctor recommended
- No garment yellowing or staining
Inactive ingredients
Water, botanical extracts, polyethylene glycol 8000, polysorbate 20, vitamin E oil, eugenol.
SWEATBLOCK Usage Instructions:
1. Apply at bedtime.
2. Dab on clean, dry skin within each underarm. (do not rub)
3. Air dry for 5 min. and go to bed.
4. In the morning, wash underarms with soap.
SWEATBLOCK Clinical Strength Antiperspirant
SWEATBLOCK
Stay Confident Longer (TM)
UP TO 8 WEEK SUPPLY*
SWEATBLOCK
Clinical Strength Antiperspirant
Stay Confident Longer (TM)
Apply every 7 days or as needed*
8 antiperspirant towelettes
*Consumer perception reports support the appearance of dryness for up to 7 days per usage. Individual results will vary based on body chemistry.
| SWEATBLOCK
aluminum chloride solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - DC Alpine Partners, LLC (042895991) |