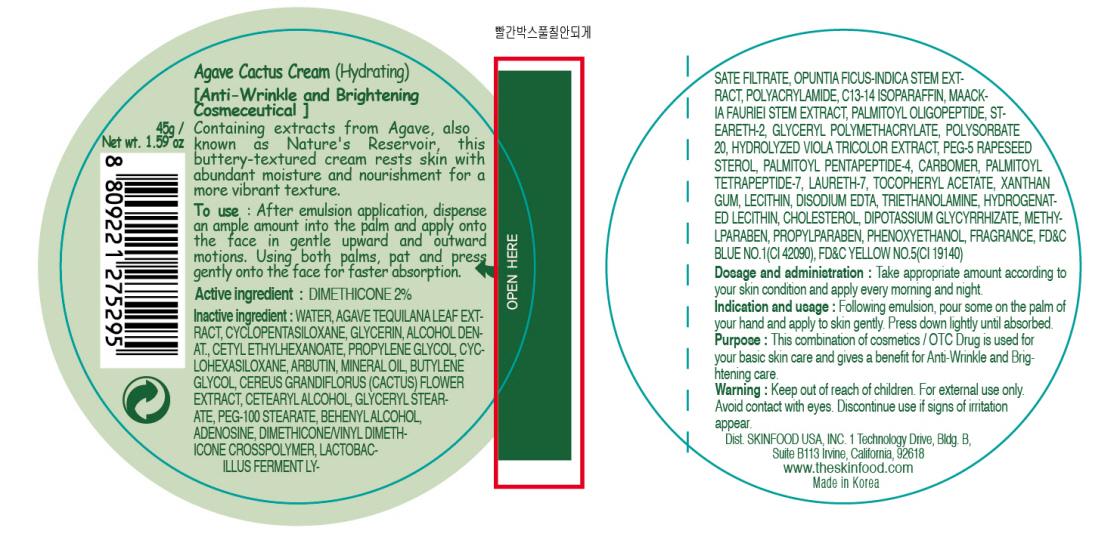
Label: AGAVE CACTUS HYDRATING- dimethicone cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 76214-006-01 - Packager: SKINFOOD CO., LTD.
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated July 22, 2011
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
-
INACTIVE INGREDIENT
Inactive ingredients:
WATER, AGAVE TEQUILANA LEAF EXTRACT, CYCLOPENTASILOXANE, GLYCERIN, ALCOHOL DENAT., CETYL ETHYLHEXANOATE, CYCLOHEXASILOXANE, PROPYLENE GLYCOL, ARBUTIN, MINERAL OIL, BUTYLENE GLYCOL, CEREUS GRANDIFLORUS (CACTUS) FLOWER EXTRACT, CETEARYL ALCOHOL, GLYCERYL STEARATE, PEG-100 STEARATE, BEHENYL ALCOHOL, ADENOSINE, DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER, LACTOBACILLUS FERMENT LYSATE FILTRATE, OPUNTIA FICUS-INDICA STEM EXTRACT, POLYACRYLAMIDE, C13-14 ISOPARAFFIN, MAACKIA FAURIEI STEM EXTRACT, PALMITOYL OLIGOPEPTIDE, STEARETH-2, GLYCERYL POLYMETHACRYLATE, POLYSORBATE 20, HYDROLYZED VIOLA TRICOLOR EXTRACT, PEG-5 RAPESEED STEROL, PALMITOYL PENTAPEPTIDE-4, CARBOMER, PALMITOYL TETRAPEPTIDE-7, LAURETH-7, TOCOPHERYL ACETATE, XANTHAN GUM, LECITHIN, DISODIUM EDTA, TRIETHANOLAMINE, HYDROGENATED LECITHIN, CHOLESTEROL, DIPOTASSIUM GLYCYRRHIZATE, METHYLPARABEN, PROPYLPARABEN, PHENOXYETHANOL, FRAGRANCE
- PURPOSE
- WARNINGS
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
- DOSAGE & ADMINISTRATION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
AGAVE CACTUS HYDRATING
dimethicone creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:76214-006 Route of Administration CUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIMETHICONE (UNII: 92RU3N3Y1O) (DIMETHICONE - UNII:92RU3N3Y1O) DIMETHICONE 0.9 g in 45 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) AGAVE TEQUILANA LEAF (UNII: 05545M0E3M) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) GLYCERIN (UNII: PDC6A3C0OX) CETYL ETHYLHEXANOATE (UNII: 134647WMX4) CYCLOMETHICONE 6 (UNII: XHK3U310BA) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) ARBUTIN (UNII: C5INA23HXF) MINERAL OIL (UNII: T5L8T28FGP) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) PEG-100 STEARATE (UNII: YD01N1999R) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) DOCOSANOL (UNII: 9G1OE216XY) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) OPUNTIA FICUS-INDICA STEM (UNII: MUD8892KHL) PHENOXYETHANOL (UNII: HIE492ZZ3T) METHYLPARABEN (UNII: A2I8C7HI9T) C13-14 ISOPARAFFIN (UNII: E4F12ROE70) MAACKIA FLORIBUNDA STEM (UNII: 84UXW52K8Y) PALMITOYL OLIGOPEPTIDE (UNII: HO4ZT5S86C) STEARETH-2 (UNII: V56DFE46J5) POLYSORBATE 20 (UNII: 7T1F30V5YH) PROPYLPARABEN (UNII: Z8IX2SC1OH) PALMITOYL PENTAPEPTIDE-4 (UNII: KK181SM5JG) PALMITOYL TETRAPEPTIDE-7 (UNII: Q41S464P1R) LAURETH-7 (UNII: Z95S6G8201) XANTHAN GUM (UNII: TTV12P4NEE) ADENOSINE (UNII: K72T3FS567) TROLAMINE (UNII: 9O3K93S3TK) EDETATE DISODIUM (UNII: 7FLD91C86K) HYDROGENATED SOYBEAN LECITHIN (UNII: H1109Z9J4N) CHOLESTEROL (UNII: 97C5T2UQ7J) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76214-006-01 45 g in 1 CARTON Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part347 03/01/2011 Labeler - SKINFOOD CO., LTD. (690324173) Registrant - SKINFOOD CO., LTD. (690324173) Establishment Name Address ID/FEI Business Operations SKINFOOD CO., LTD. 690324173 manufacture