Label: LORATADINE tablet
- NDC Code(s): 51079-246-01, 51079-246-20
- Packager: Mylan Institutional Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated April 11, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient (in each tablet)
- Purpose
- Uses
-
Warnings
Ask a doctor before use if you have
liver or kidney disease. Your doctor should determine if you need a different dose.
When using this product
do not take more than directed. Taking more than directed may cause drowsiness.
- Directions (24 Hour Relief)
- Other information
- Inactive Ingredients
-
Questions or comments?
1-800-848-0462
- Serious side effects associated with use of this product may be reported to this number.
Manufactured for:
Mylan Pharmaceuticals Inc.
Morgantown, WV 26505 U.S.A.Made in India
Code No.: MH/DRUGS/25/NKD/89
Distributed by:
Mylan Institutional Inc.
Rockford, IL 61103 U.S.A.S-11333 R2
11/16 -
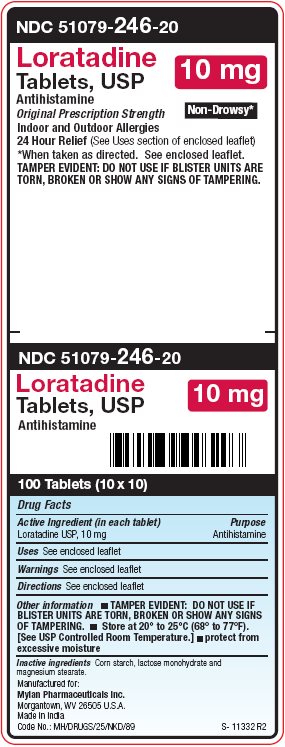
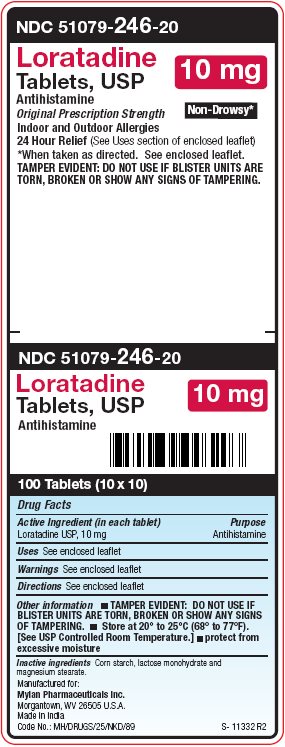
PRINCIPAL DISPLAY PANEL – 10 mg
NDC 51079-246-20
Loratadine
Tablets, USP
10 mgAntihistamine
Original Prescription Strength Non-Drowsy*
Indoor and Outdoor Allergies
24 Hour Relief (See Uses section of enclosed leaflet)
*When taken as directed. See enclosed leaflet.
TAMPER EVIDENT: DO NOT USE IF BLISTER UNITS ARE
TORN, BROKEN OR SHOW ANY SIGNS OF TAMPERING. -
INGREDIENTS AND APPEARANCE
LORATADINE
loratadine tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51079-246 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LORATADINE (UNII: 7AJO3BO7QN) (LORATADINE - UNII:7AJO3BO7QN) LORATADINE 10 mg Inactive Ingredients Ingredient Name Strength STARCH, CORN (UNII: O8232NY3SJ) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) Product Characteristics Color white (white to off-white) Score no score Shape ROUND Size 6mm Flavor Imprint Code G;L;10 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51079-246-20 100 in 1 BOX, UNIT-DOSE 04/30/2013 08/31/2024 1 NDC:51079-246-01 1 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA076154 04/30/2013 08/31/2024 Labeler - Mylan Institutional Inc. (039615992)