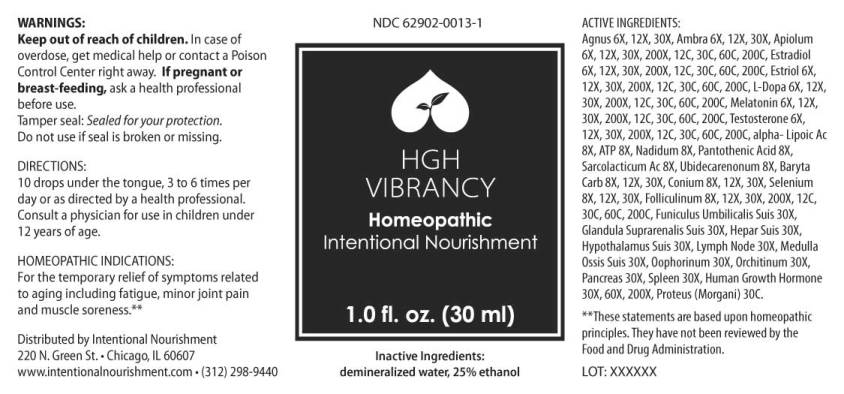
HGH VIBRANCY- agnus castus, ambra grisea, conium maculatum, apiolum, estradiol, estriol, folliculinum, l-dopa, melatonin, testosterone, alpha-lipoicum acidum, adenosinum triphosphoricum dinatrum, ubidecarenonum, nadidum, sarcolacticum acidum, baryta carbonica, selenium metallicum, funiculus umbilicalis suis, glandula suprarenalis suis, hepar suis, hypothalamus (suis), lymph node(suis), medulla ossis suis, oophorinum (suis), orchitinum (suis), pancreas suis, spleen (suis), human growth hormone, proteus (morgani) liquid
Intentional Nourishment
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
Drug Facts:
ACTIVE INGREDIENTS:
Agnus Castus 6X, 12X, 30X, Ambra Grisea 6X, 12X, 30X, Conium Maculatum 6X, 12X, 30X, Apiolum 6X, 12X, 30X, 200X, 12C, 30C , 60C, 200C, Estradiol 6X, 12X, 30X, 200X, 12C, 30C, 60C, 200C, Estriol 6X, 12X, 30X, 200X, 12C, 30C, 60C, 200C, Folliculinum 6X, 12X, 30X, 200X, 12C, 30C, 60C, 200C, L-Dopa 6X, 12X, 30X, 200X, 12C, 30C, 60C, 200C, Melatonin 6X, 12X, 30X, 200X, 12C, 30C, 60C, 200C, Testosterone 6X, 12X, 30X, 200X, 12C, 30C, 60C, 200C, Alpha-Lipoicum Acidum 8X, Adenosinum Triphosphoricum Dinatrum 8X, Nadidum 8X, Pantothenic Acid 8X, Sarcolacticum Acidum 8X, Ubidecarenonum 8X, Baryta Carbonica 8X, 12X, 30X, Selenium Metallicum 8X, 12X, 30X, Funiculus Umbilicalis Suis 30X, Glandula Suprarenalis Suis 30X, Hepar Suis 30X, Hypothalamus Suis 30X, Lymph Node (Suis) 30X, Medulla Ossis Suis 30X, Oophorinum (Suis) 30X, Orchitinum (Suis) 30X, Pancreas Suis 30X, Spleen (Suis) 30X, Human Growth Hormone 30X, 60X, 200X, Proteus (Morgani) 30C.
INDICATIONS:
For the temporary relief of symptoms related to aging including fatigue, minor joint pain and muscle soreness.**
**These statements are based upon homeopathic principles. They have not been reviewed by the Food and Drug Administration.
WARNINGS:
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
If pregnant or breast-feeding, ask a health professional before use.
Tamper seal: Sealed for your protection.
Do not use if seal is broken or missing.
KEEP OUT OF REACH OF CHILDREN:
Keep out of reach of children. In case of overdose, contact physician or Poison Control Center right away.
DIRECTIONS:
10 drops under the tongue, 3 to 6 times per day or as directed by a health professional. Consult a physician for use in children under 12 years of age.
INDICATIONS:
For the temporary relief of symptoms related to aging including fatigue, minor joint pain and muscle soreness.**
**These statements are based upon homeopathic principles. They have not been reviewed by the Food and Drug Administration.
| HGH VIBRANCY
agnus castus, ambra grisea, conium maculatum, apiolum, estradiol, estriol, folliculinum, l-dopa, melatonin, testosterone, alpha-lipoicum acidum, adenosinum triphosphoricum dinatrum, ubidecarenonum, nadidum, sarcolacticum acidum, baryta carbonica, selenium metallicum, funiculus umbilicalis suis, glandula suprarenalis suis, hepar suis, hypothalamus (suis), lymph node(suis), medulla ossis suis, oophorinum (suis), orchitinum (suis), pancreas suis, spleen (suis), human growth hormone, proteus (morgani) liquid |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Intentional Nourishment (036906156) |
| Registrant - Apotheca Company (844330915) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Apotheca Company | 844330915 | manufacture(62902-0013) , api manufacture(62902-0013) , label(62902-0013) , pack(62902-0013) | |