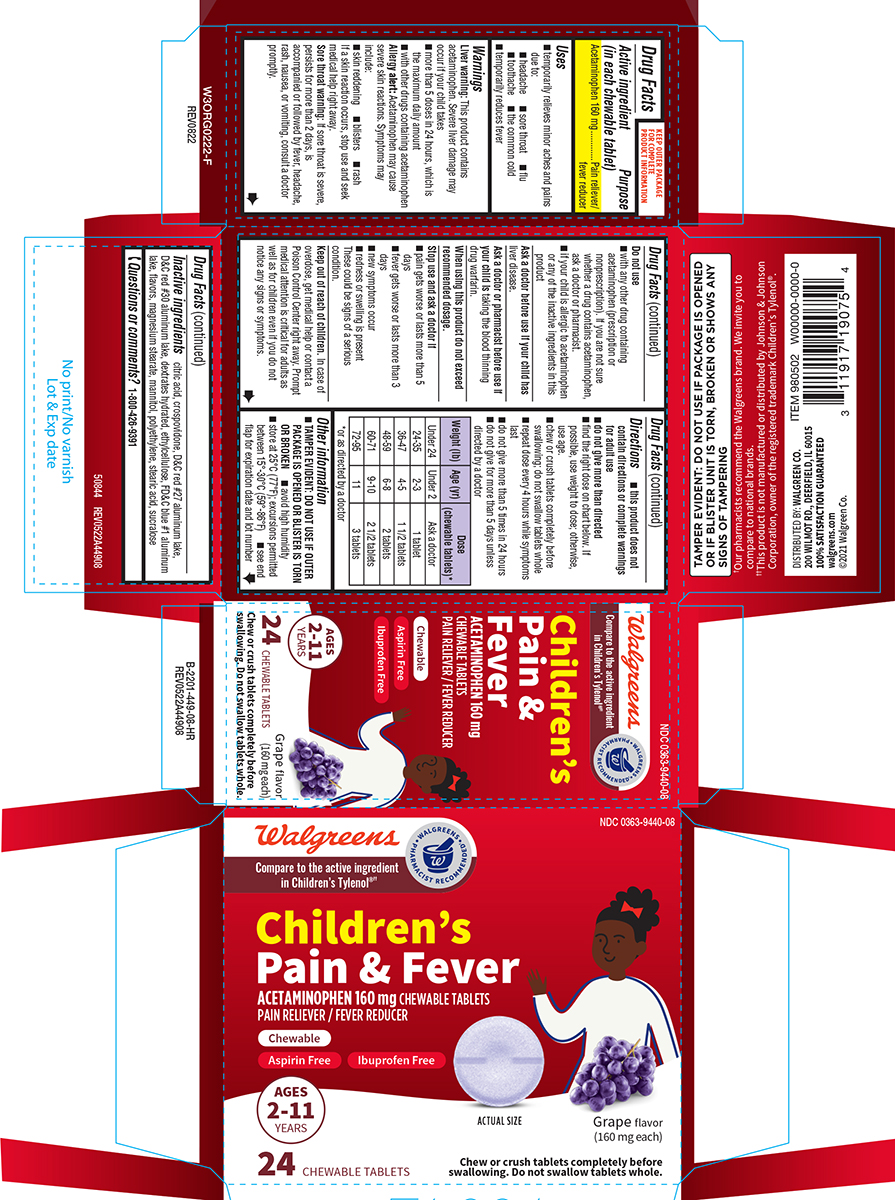
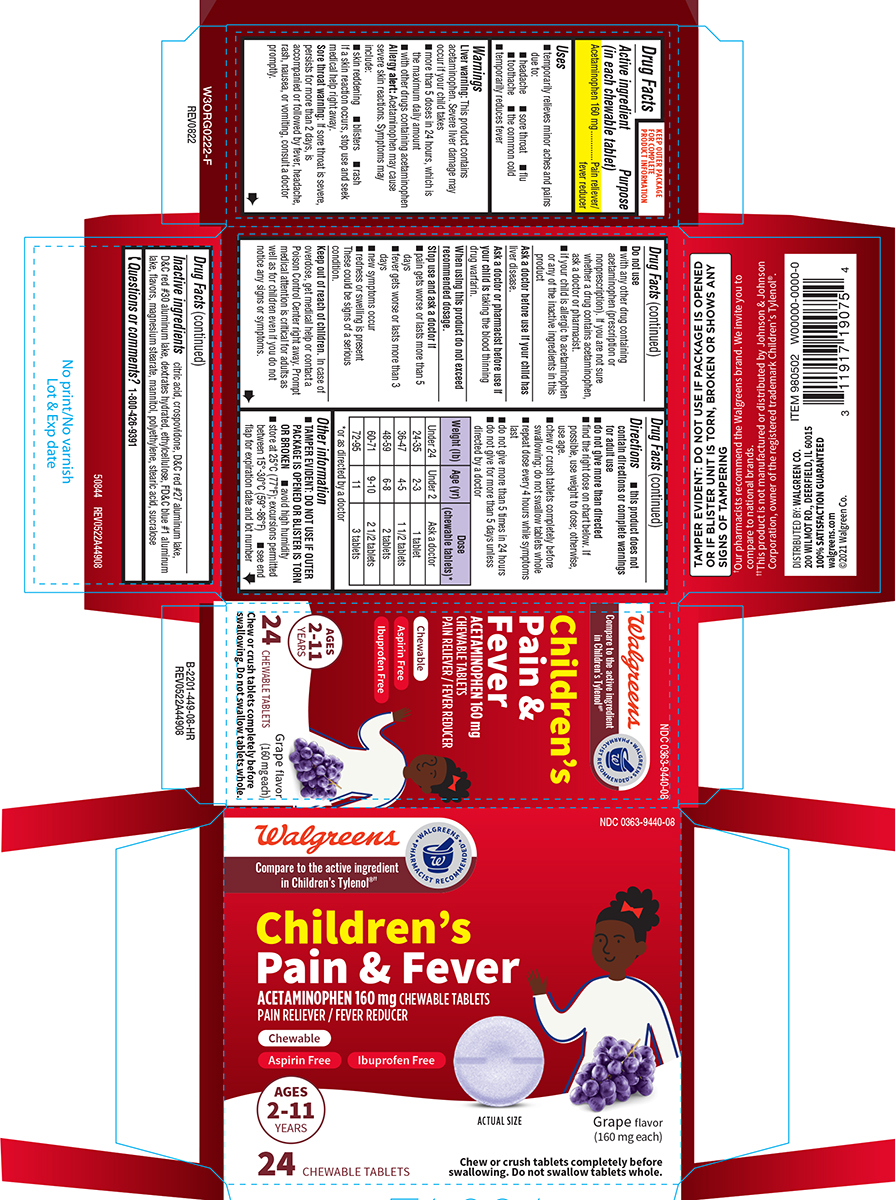
Label: PAIN AND FEVER CHILDRENS- acetaminophen tablet, chewable
- NDC Code(s): 0363-9440-08
- Packager: Walgreen Company
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated January 26, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient (in each chewable tablet)
- Purpose
- Uses
-
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if your child takes
- more than 5 doses in 24 hours, which is the maximum daily amount
- with other drugs containing acetaminophen
Allergy alert: Acetaminophen may cause severe skin reactions. Symptoms may include:
- skin reddening
- blisters
- rash
If a skin reaction occurs, stop use and seek medical help right away.
Sore throat warning: If sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea, or vomiting, consult a doctor promptly.
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- if your child is allergic to acetaminophen or any of the inactive ingredients in this product
-
Directions
- this product does not contain directions or complete warnings for adult use
- do not give more than directed
- find the right dose on chart below. If possible, use weight to dose; otherwise, use age.
- chew or crush tablets completely before swallowing; do not swallow tablets whole
- repeat dose every 4 hours while symptoms last
- do not give more than 5 times in 24 hours
- do not give for more than 5 days unless directed by a doctor
Weight (lb) Age (yr)
Dose (chewable tablets)*
Under 24
Under 2
Ask a doctor
24-35
2-3
1 tablet
36-47
4-5
1 1/2 tablets
48-59
6-8
2 tablets
60-71
9-10
2 1/2 tablets
72-95
11
3 tablets
*or as directed by a doctor
- Other information
- Inactive ingredients
- Questions or comments?
-
Principal display panel
NDC 0363-9440-08
Walgreens
Compare to the active ingredient
in Children’s Tylenol®††• WALGREENS •
PHARMACIST RECOMMENDED†Children’s
Pain & FeverACETAMINOPHEN 160 mg CHEWABLE TABLETS
PAIN RELIEVER / FEVER REDUCERChewable
Aspirin Free
Ibuprofen FreeAGES
2-11
YEARS24 CHEWABLE TABLETS
ACTUAL SIZE Grape flavor (160 mg each)
Chew or crush tablets completely before
swallowing. Do not swallow tablets whole.TAMPER EVIDENT: DO NOT USE IF PACKAGE IS OPENED
OR IF BLISTER UNIT IS TORN, BROKEN OR SHOWS ANY
SIGNS OF TAMPERINGWalgreens Pharmacist Recommended
Walgreens Pharmacist Survey
†Our pharmacists recommend the Walgreens brand. We invite you to
compare to national brands.
††This product is not manufactured or distributed by Johnson & Johnson
Corporation, owner of the registered trademark Children’s Tylenol®.
DISTRIBUTED BY: WALGREEN CO.
200 WILMOT RD., DEERFIELD, IL 60015
100% SATISFACTION GUARANTEED
walgreens.com
©2021 Walgreen Co.50844 REV0522A44908
Walgreens 44-449
-
INGREDIENTS AND APPEARANCE
PAIN AND FEVER CHILDRENS
acetaminophen tablet, chewableProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0363-9440 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 160 mg Inactive Ingredients Ingredient Name Strength CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) CROSPOVIDONE, UNSPECIFIED (UNII: 2S7830E561) D&C RED NO. 27 ALUMINUM LAKE (UNII: ZK64F7XSTX) D&C RED NO. 30 (UNII: 2S42T2808B) DEXTROSE MONOHYDRATE (UNII: LX22YL083G) ETHYLCELLULOSE, UNSPECIFIED (UNII: 7Z8S9VYZ4B) FD&C BLUE NO. 1 ALUMINUM LAKE (UNII: J9EQA3S2JM) MAGNESIUM STEARATE (UNII: 70097M6I30) MANNITOL (UNII: 3OWL53L36A) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) STEARIC ACID (UNII: 4ELV7Z65AP) SUCRALOSE (UNII: 96K6UQ3ZD4) Product Characteristics Color purple Score 2 pieces Shape ROUND Size 16mm Flavor GRAPE Imprint Code 44;449 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0363-9440-08 4 in 1 CARTON 01/28/2005 1 6 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M013 01/28/2005 Labeler - Walgreen Company (008965063) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 038154464 pack(0363-9440) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 832867837 manufacture(0363-9440) , pack(0363-9440) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 967626305 pack(0363-9440) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 117025878 manufacture(0363-9440)