ASTRAZENECA COVID-19 VACCINE- azd1222 injection, suspension
AstraZeneca Pharmaceuticals LP
----------
AstraZeneca COVID-19 Vaccine
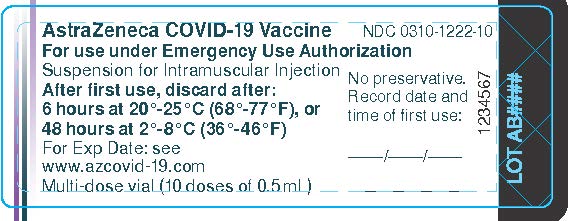
Package/Label Display Panel – Vial Label
AstraZeneca COVID-19 Vaccine NDC 0310-1222-10
For use under Emergency Use Authorization
Suspension for Intramuscular Injection
After first use, discard after:
6 hours at 20°-25°C (68°-77°F), or
48 hours at 2°-8°C (36°-46°F)
For Exp Date: see
Multi-dose vial (10 doses of 0.5 mL)
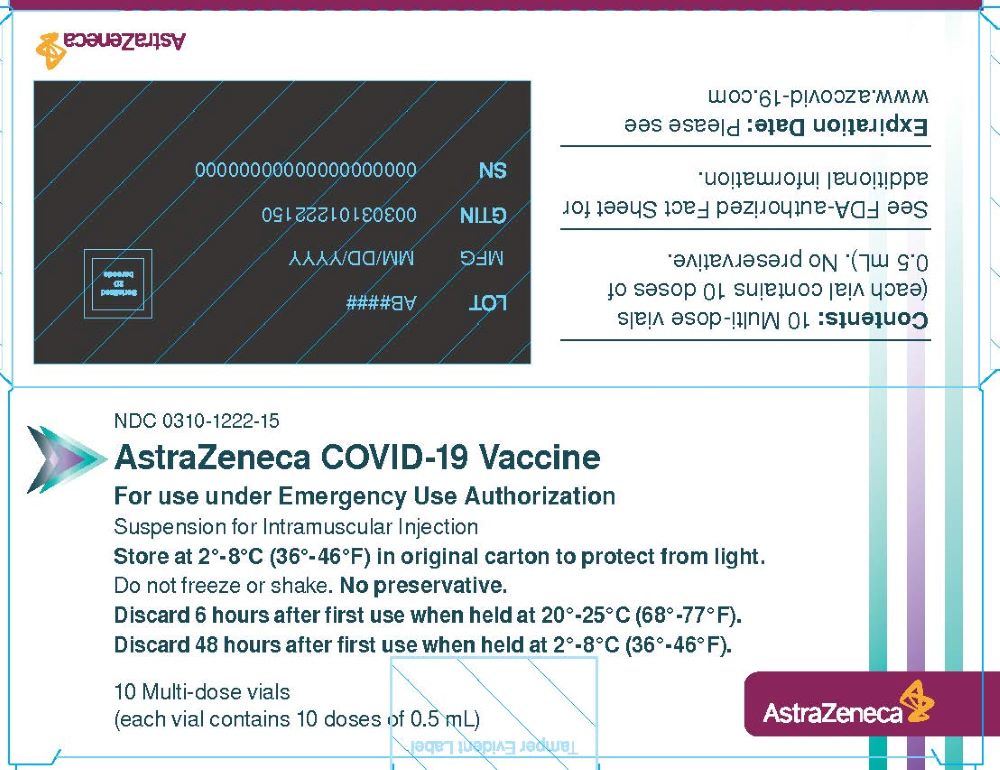
Package/Label Display Panel – Vial Carton
NDC 0310-1222-15
AstraZeneca COVID-19 Vaccine
For use under Emergency Use Authorization
Suspension for Intramuscular Injection
Store at 2°-8°C (36°-46°F) in original carton to protect from light.
Do not freeze or shake. No preservative.
Discard 6 hours after first use when held at 20°-25°C (68°-77°F).
Discard 48 hours after first use when held at 2°-8°C (36°-46°F).
10 Multi-dose vials
(each vial contains 10 doses of 0.5 mL)
AstraZeneca
| ASTRAZENECA COVID-19 VACCINE
azd1222 injection, suspension |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - AstraZeneca Pharmaceuticals LP (054743190) |
| Registrant - AstraZeneca PLC (230790719) |