Label: PHENAZOPYRIDINE HYDROCHLORIDE tablet, coated
-
Contains inactivated NDC Code(s)
NDC Code(s): 73028-201-01, 73028-202-01 - Packager: Pageview Pharmaceuticals
- Category: HUMAN PRESCRIPTION DRUG LABEL
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated January 18, 2022
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
Phenazopyridine Hydrochloride is a red-brown, odorless, slightly bitter, crystalline powder. It has a specific local analgesic effect in the urinary tract, promptly relieving burning and pain.
Structural formula:
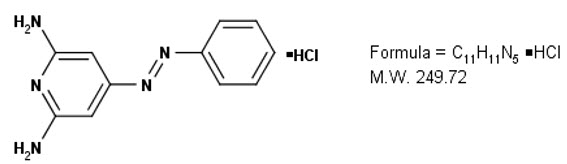
Phenazopyridine HCl oral tablets contain the following inactive ingredients: Calcium Phosphate, Sodium Starch Glycolate and Magnesium Stearate.
- CLINICAL PHARMACOLOGY
-
INDICATIONS AND USAGE
Phenazopyridine HCl is indicated for the symptomatic relief of pain, burning, urgency frequency, and other discomforts arising from irritation of the mucosa of the lower urinary tract caused by infection, trauma, surgery, endoscopic procedures, or the passage of sounds or catheters.
The use of phenazopyridine for relief of symptoms should not delay definitive diagnosis and treatment of causative conditions. The drug should be used for symptomatic relief of pain and not as a substitute for specific surgery or antimicrobial therapy.
Phenazopyridine is compatible with antimicrobial therapy and can help relieve pain and discomfort during the interval before antimicrobial therapy controls the infection.
Treatment of a urinary tract infection with phenazopyridine should not exceed 2 days. There is no evidence that the combined administration of phenazopyridine and an antimicrobial provides greater benefit than administration of the antimicrobial alone after 2 days. (See Dosage and Administration.)
- CONTRAINDICATIONS
-
PRECAUTIONS
The patient should be advised that phenazopyridine produces an orange to red color in the urine and feces, and may cause staining. Phenazopyridine may cause discoloration of body fluids and staining of contact lenses has been reported. A yellowish color of the skin or sclera may indicate accumulation of phenazopyridine resulting from impaired renal function and necessitates discontinuance of the drug. It should be noted that a decline in renal function is common in elderly patients. Phenazopyridine may mask pathological conditions and interfere with laboratory test values using colorimetric, spectrophotometric or fluorometric analysis methods.
Cautious use in patients with G-6-PD deficiency is advised since these patients are susceptible to oxidative hemolysis and may have greater potential to develop hemolytic anemia.
Information for Patients
The patient should be advised to take phenazopyridine with or following food or after eating a snack to reduce stomach upset.
The patients should be aware that phenazopyridine causes a reddish orange discoloration of the urine and feces, and may stain clothing. Phenazopyridine may cause discoloration of body fluids and staining of contact lenses has been reported. There have been reports of teeth discoloration when the product has been broken or held in the mouth prior to swallowing.
Patients should be instructed to take phenazopyridine for only 2 days if an antibacterial agent is administered concurrently for the treatment of a urinary tract infection. If symptoms persist beyond those 2 days, the patient should be instructed to contact his or her physician.
Laboratory Tests
Phenazopyridine may interfere with laboratory test values using colorimetric, photometric or fluorometric analysis methods.
Altered urine laboratory test values may include ketone (sodium nitroprusside), bilirubin (foam test, talc-disk-Fouchet-spot test, Franklin's tablet-Fouchet test, p-nitrobenzene diazonium p-toluene sulfonate reagent), diacetic acid (Gerhardt ferric chloride test), free hydrochloric acid, glucose (glucose oxidase tests), 17-hydroxycorticosteroids (modified Glenn-Nelson), 17-ketosteroids (Holtorff Koch modification of Zimmerman), porphyrins, albumin (discolors bromophenol blue test areas of commercial reagent strips, nitric acid ring test), phenolsulfophthalein, urobilinogen (color interference with Ehrlich's reagent), and urinalysis (spectrophotometric or color-based tests). Phenazopyridine also imparts an orange-red color to stools which may interfere with color tests.
Drug Interactions
The interaction of phenazopyridine with other drugs has not been studied in a systematic manner. However, the medical literature to date suggests that no significant interactions have been reported Carcinogenesis, Mutagenesis, Impairment of Fertility:
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term administration of phenazopyridine has been associated with tumors of the large intestine in rats and of the liver in mice. Available epidemiological data are insufficient to evaluate the carcinogenicity of phenazopyridine in humans. In vitro studies indicate that phenazopyridine in the presence of metabolic activation is mutagenic in bacteria and mutagenic and clastogenic in mammalian cells.
Pregnancy Category B
Reproductive studies with phenazopyridine (in combination with sulfacytine) in rats given up to 110 mg/kg/day and in rabbits given up to 39 mg/kg/day during organogenesis revealed no evidence of harm to offspring.
One prospective study in humans demonstrated that phenazopyridine traverses the placenta into the fetal compartment. There are no adequate and well-controlled studies in pregnant women. Therefore, phenazopyridine should be used in pregnant women only if the benefit clearly outweighs the risk.
-
ADVERSE REACTIONS
The following adverse events have been reported:
CNS: headache.
Gastrointestinal: nausea, vomiting and diarrhea.
Dermatologic and Hypersensitivity: rash, pruritus, discoloration, anaphylactoid-like reaction and hypersensitivity hepatitis
Hematologic: methemoglobinemia, hemolytic anemia, potential hemolytic agent in G-6-PD deficiency, sulfhemoglobinemia.
Other: visual disturbances, renal and hepatic toxicity usually associated with overdose, renal calculi, jaundice, discoloration of body fluids and aseptic meningitis.
-
OVERDOSAGE
Symptoms: Exceeding the recommended dose in patients with normal renal function or administering the recommended dose to patients with impaired renal function (common in elderly patients) may lead to increased serum levels and toxic reactions. Methemoglobinemia generally follows a massive, acute overdose. Methylene blue, 1 to 2 mg/kg/dose given intravenously as a 1% solution as needed, should cause prompt reduction of the methemoglobinemia and disappearance of the cyanosis which is an aid in diagnosis. Oxidative Heinz body hemolytic anemia also may occur, and "bite cells" (degmacytes) may be present in a chronic overdosage situation. Red blood cell G-6-PD deficiency may predispose to hemolysis; however, hemolysis may occur at normal doses in patients with G-6-PD Mediterranean.
Renal toxicity and occasional failure and hepatic impairment may also occur.
Treatment: Treatment is symptomatic and supportive.
- DOSAGE AND ADMINISTRATION
-
HOW SUPPLIED
100 mg Tablets: Supplied in bottles of 100 count NDC# 73028-201-01.
Appearance: Reddish-brown, round tablets debossed "C1" on one side and plain on the reverse side.
200 mg Tablets: Supplied in bottles of 100 count NDC# 73028-202-01.
Appearance: Redish-brown, round tablets debossed "C7" on one side and plain on the reverse side.
-
SPL UNCLASSIFIED SECTION
PHARMACIST: This product is not an Orange Book rated product, therefore all prescriptions using this product shall be subject to state and federal statutes as applicable. This product has not been subjected to FDA therapeutic or other equivalency testing. There are no claims of bioequivalence or therapeutic equivalence. Each person recommending a prescription substitution using this product shall make such recommendation based on his/her professional knowledge and opinion, upon evaluating the active ingredients, inactive ingredients, excipients and chemical information contained within the enclosed prescribing information.
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 100 mg Tablet Bottle Label
- PRINCIPAL DISPLAY PANEL - 200 mg Tablet Bottle Label
-
INGREDIENTS AND APPEARANCE
PHENAZOPYRIDINE HYDROCHLORIDE
phenazopyridine hydrochloride tablet, coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:73028-201 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength phenazopyridine hydrochloride (UNII: 0EWG668W17) (PHENAZOPYRIDINE - UNII:K2J09EMJ52) phenazopyridine hydrochloride 100 mg Product Characteristics Color ORANGE (Orange-red) Score no score Shape ROUND Size 4mm Flavor Imprint Code C1 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73028-201-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 12/01/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date UNAPPROVED DRUG OTHER 12/01/2020 PHENAZOPYRIDINE HYDROCHLORIDE
phenazopyridine hydrochloride tablet, coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:73028-202 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength phenazopyridine hydrochloride (UNII: 0EWG668W17) (PHENAZOPYRIDINE - UNII:K2J09EMJ52) phenazopyridine hydrochloride 200 mg Product Characteristics Color ORANGE (Orange-red) Score no score Shape ROUND Size 10mm Flavor Imprint Code C7 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73028-202-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 12/01/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date UNAPPROVED DRUG OTHER 12/01/2020 Labeler - Pageview Pharmaceuticals (117003305)




