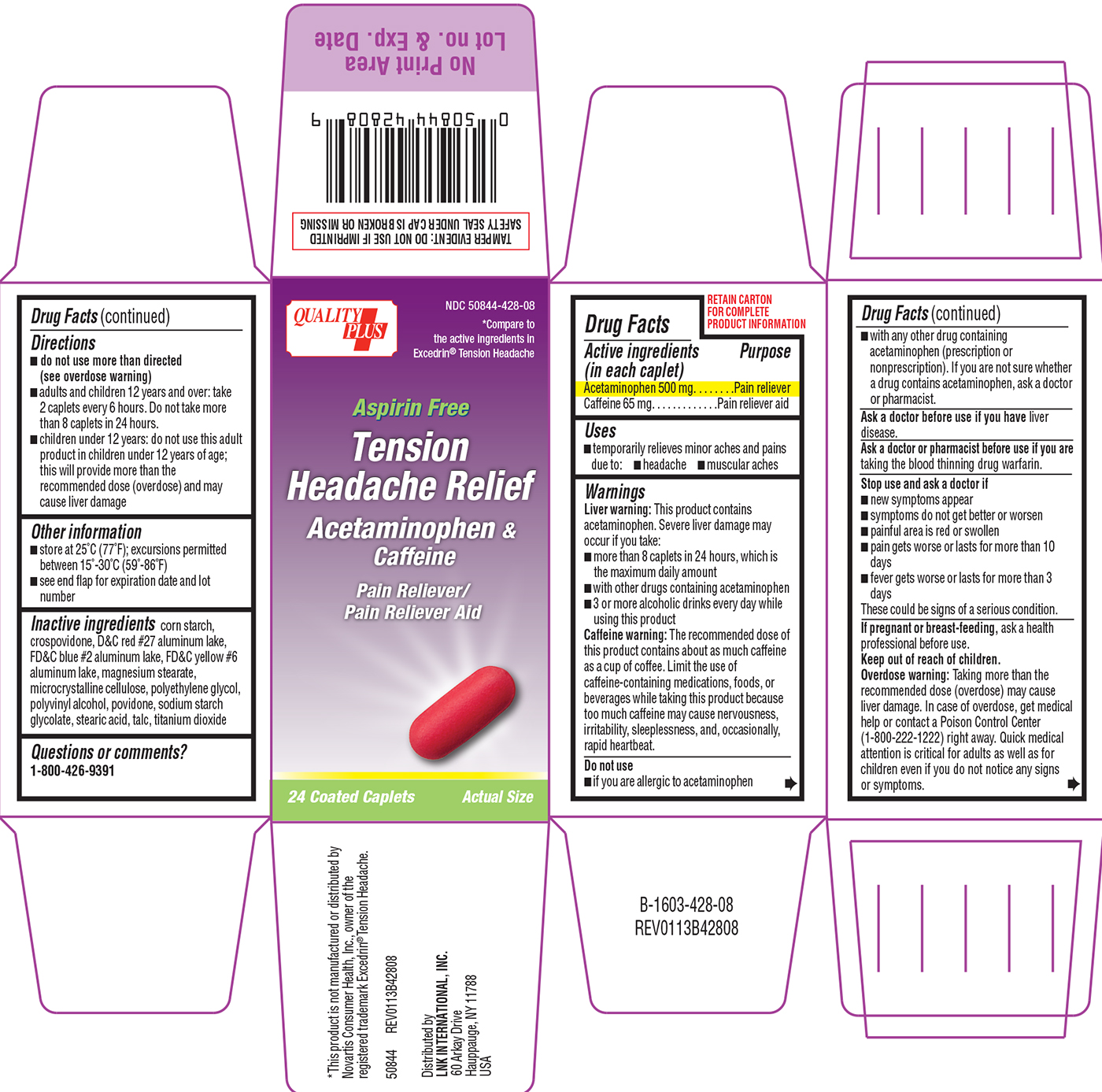
TENSION HEADACHE RELIEF ASPIRIN FREE- acetaminophen, caffeine tablet, film coated
L.N.K. International, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Quality Plus 44-428-Delisted
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take:
- more than 8 caplets in 24 hours, which is the maximum daily amount
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks every day while using this product
Caffeine warning: The recommended dose of this product contains about as much caffeine as a cup of coffee. Limit the use of caffeine-containing medications, foods, or beverages while taking this product because too much caffeine may cause nervousness, irritability, sleeplessness, and, occasionally, rapid heartbeat.
Do not use
- if you are allergic to acetaminophen
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
Stop use and ask a doctor if
- new symptoms appear
- symptoms do not get better or worsen
- painful area is red and swollen
- pain gets worse or lasts more than 10 days
- fever gets worse or lasts more than 3 days
These could be signs of a serious condition.
Keep out of reach of children.
Overdose warning: Taking more than the recommended dose (overdose) may cause liver damage. In case of overdose, get medical help or contact a Poison Control Center (1-800-222-1222) right away. Quick medical attention is critical for adults as well as for children even if you do not notice any signs or symptoms.
Directions
- do not use more than directed (see overdose warning)
- adults and children 12 years and over: take 2 caplets every 6 hours. Do not take more than 8 caplets in 24 hours.
- children under 12 years: do not use this adult product in children under 12 years of age; this will provide more than the recommended dose (overdose) and may cause liver damage
Other information
- store at 25ºC (77ºF); excursions permitted between 15º-30ºC (59º-86ºF)
- see end flap for expiration date and lot number
Inactive ingredients
corn starch, crospovidone, D&C red #27 aluminum lake, FD&C blue #2 aluminum lake, FD&C yellow #6 aluminum lake, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polyvinyl alcohol, povidone, sodium starch glycolate, stearic acid, talc, titanium dioxide
Principal display panel
QUALITY PLUS
NDC 50844-428-08
*Compare to the active ingredients in Excedrin® Tension Headache
Aspirin Free
Tension Headache Relief
Acetaminophen & Caffeine
Pain Reliever/Pain Reliever Aid
24 Coated Caplets
Actual Size
*This product is not manufactured or distributed by Novartis Consumer Health, Inc., owner of the registered trademark Excedrin® Tension Headache.
50844 REV0113B42808
Distributed by
LNK INTERNATIONAL, INC.
60 Arkay Drive
Hauppauge, NY 11788
USA
TAMPER EVIDENT: DO NOT USE IF IMPRINTED SAFETY SEAL UNDER CAP IS BROKEN OR MISSING
Quality Plus 44-428
| TENSION HEADACHE RELIEF
ASPIRIN FREE
acetaminophen, caffeine tablet, film coated |
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
| Labeler - L.N.K. International, Inc. (038154464) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 038154464 | pack(50844-428) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 832867894 | manufacture(50844-428) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 967626305 | pack(50844-428) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 868734088 | pack(50844-428) | |