EVEKEO ODT- amphetamine sulfate tablet, orally disintegrating
Azurity Pharmaceuticals, Inc.
----------
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use EVEKEO ODT
®safely and effectively. See full prescribing information for EVEKEO ODT.
EVEKEO ODT (amphetamine sulfate) orally disintegrating tablets, CII Initial U.S. Approval: 1984 WARNING: ABUSE, MISUSE, AND ADDICTIONSee full prescribing information for complete boxed warning.EVEKEO ODT has a high potential for abuse and misuse, which can lead to the development of a substance use disorder, including addiction. Misuse and abuse of CNS stimulants, including EVEKEO ODT, can result in overdose and death ( 5.1, 9.2, 10):
RECENT MAJOR CHANGESINDICATIONS AND USAGEEVEKEO ODT is a central nervous system (CNS) stimulant indicated for the treatment of Attention Deficit Hyperactivity Disorder (ADHD) in pediatric patients 6 to 17 years of age. ( 1) DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHSOrally disintegrating tablets: 5 mg, 10 mg, 15 mg, and 20 mg. ( 3) CONTRAINDICATIONSWARNINGS AND PRECAUTIONS
ADVERSE REACTIONSMost common adverse reactions (incidence ≥4% and at a rate at least twice placebo) in pediatric patients are: decreased appetite and insomnia. ( 6) To report SUSPECTED ADVERSE REACTIONS, contact Arbor Pharmaceuticals, LLC at 1-800-461-7449 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. DRUG INTERACTIONSAcidifying and Alkalinizing Agents: Agents that alter GI and urinary pH can alter blood levels of amphetamine. Acidifying agents (GI and urinary) can decrease amphetamine blood levels, while alkalinizing agents (GI and urinary) can increase amphetamine blood levels. Adjust EVEKEO ODT dosage accordingly. ( 2.5, 7.1) USE IN SPECIFIC POPULATIONSSee 17 for PATIENT COUNSELING INFORMATION and Medication Guide. Revised: 10/2023 |
FULL PRESCRIBING INFORMATION
WARNING: ABUSE, MISUSE, AND ADDICTION
EVEKEO ODT has a high potential for abuse and misuse, which can lead to the development of a substance use disorder, including addiction. Misuse and abuse of CNS stimulants, including EVEKEO ODT, can result in overdose and death [see Overdosage (10)] , and this risk is increased with higher doses or unapproved methods of administration, such as snorting or injection.
Before prescribing EVEKEO ODT, assess each patient's risk for abuse, misuse, and addiction. Educate patients and their families about these risks, proper storage of the drug, and proper disposal of any unused drug. Throughout EVEKEO ODT treatment, reassess each patient's risk of abuse, misuse, and addiction and frequently monitor for signs and symptoms of abuse, misuse, and addiction [see Warnings and Precautions (5.1)and Drug Abuse and Dependence (9.2)].
1 INDICATIONS AND USAGE
EVEKEO ODT is indicated for the treatment of Attention Deficit Hyperactivity Disorder (ADHD) in pediatric patients 6 to 17 years of age.
2 DOSAGE AND ADMINISTRATION
2.1 Pretreatment Screening
Prior to treating patients with EVEKEO ODT, assess:
- for the presence of cardiac disease (i.e., perform a careful history, family history of sudden death or ventricular arrhythmia, and physical exam) [see Warnings and Precautions (5.2)].
- the family history and clinically evaluate patients for motor or verbal tics or Tourette's syndrome before initiating EVEKEO ODT [see Warnings and Precautions (5.9)].
2.2 Recommended Dosage
Administer EVEKEO ODT orally in the morning with or without food or liquid.
The recommended starting dosage for pediatric patients 6 to 17 years of age is 5 mg once or twice daily. If necessary, administer an additional dose after 4 to 6 hours. Titrate the dosage in increments of 5 mg at weekly intervals depending on response and tolerability. Only in rare cases will it be necessary to exceed a total of 40 mg daily.
Amphetamine should be administered at the lowest effective dosage and dosage should be individually adjusted.
2.3 Administration Instructions
Instruct the patient or caregiver on the following administration instructions:
- Do not remove the tablet from the blister pack until just prior to dosing. Do not store the tablet for future use.
- Use dry hands to open the blister.
- Remove the tablet by pushing it through the back of the foil-lined blister pack.
- As soon as the blister is opened, remove the tablet and place the tablet on the patient's tongue.
- Place the whole tablet on the tongue and allow it to disintegrate without chewing or crushing.
- The tablet will disintegrate in saliva so that it can be swallowed. No liquid is needed to take the tablet. The tablet can be actively moved around between the tongue and the roof of the mouth until it disintegrates.
2.4 Switching from Other Amphetamine Products
Switching from EVEKEO to EVEKEO ODT can be done on a milligram-per-milligram basis.
When switching from other amphetamine products, discontinue treatment and titrate with EVEKEO ODT using the titration schedule above. Do not substitute for other amphetamine products on a milligram-per-milligram basis because of different amphetamine salt compositions and differing pharmacokinetic profiles [see Description (11), Clinical Pharmacology (12.3)].
2.5 Dosage Modifications Due to Drug Interactions
Agents that alter urinary pH can impact urinary excretion and alter blood levels of amphetamine. Acidifying agents (e.g., ascorbic acid) decrease blood levels, while alkalinizing agents (e.g., sodium bicarbonate) increase blood levels. Adjust EVEKEO ODT dosage accordingly [see Drug Interactions (7.1)] .
3 DOSAGE FORMS AND STRENGTHS
EVEKEO ODT (amphetamine sulfate) orally disintegrating tablets are supplied as follows:
- 5 mg: white to off-white, round, flat-faced radius-edged tablet with "5" on one side and "EVI" on the other.
- 10 mg: white to off-white, round, flat-faced radius-edged tablet with "10" on one side and "EVI" on the other.
- 15 mg: white to off-white, round, flat-faced radius-edged tablet with "15" on one side and "EVI" on the other.
- 20 mg: white to off-white, round, flat-faced radius-edged tablet with "20" on one side and "EVI" on the other.
4 CONTRAINDICATIONS
EVEKEO ODT is contraindicated in patients:
- With known hypersensitivity to amphetamine, or other components of EVEKEO ODT. Hypersensitivity reactions such as angioedema and anaphylactic reactions have been reported in patients treated with other amphetamine products [see Adverse Reactions (6.2)] .
- Receiving concomitant treatment with monoamine oxidase inhibitors (MAOIs), or within 14 days following discontinuation of treatment with an MAOI (including MAOIs such as linezolid or intravenous methylene blue), because of an increased risk of hypertensive crisis [see Warnings and Precautions (5.8), Drug Interactions (7.1)].
5 WARNINGS AND PRECAUTIONS
5.1 Abuse, Misuse, and Addiction
EVEKEO ODT has a high potential for abuse and misuse. The use of EVEKEO ODT exposes individuals to the risks of abuse and misuse, which can lead to the development of a substance use disorder, including addiction. EVEKEO ODT can be diverted for non-medical use into illicit channels or distribution [see Drug Abuse and Dependence (9.2)]. Misuse and abuse of CNS stimulants, including EVEKEO ODT, can result in overdose and death [see Overdosage (10)] , and this risk is increased with higher doses or unapproved methods of administration, such as snorting or injection.
Before prescribing EVEKEO ODT, assess each patient's risk for abuse, misuse, and addiction. Educate patients and their families about these risks and proper disposal of any unused drug. Advise patients to store EVEKEO ODT in a safe place, preferably locked, and instruct patients to not give EVEKEO ODT to anyone else. Throughout EVEKEO ODT treatment, reassess each patient's risk of abuse, misuse, and addiction and frequently monitor for signs and symptoms of abuse, misuse, and addiction.
5.2 Risks to Patients with Serious Cardiac Disease
Sudden death has been reported in patients with structural cardiac abnormalities or other serious cardiac disease who were treated with CNS stimulants at the recommended ADHD dosage.
Avoid EVEKEO ODT use in patients with known structural cardiac abnormalities, cardiomyopathy, serious cardiac arrhythmia, coronary artery disease, or other serious cardiac disease.
5.3 Increased Blood Pressure and Heart Rate
CNS stimulants cause an increase in blood pressure (mean increase about 2 to 4 mm Hg) and heart rate (mean increase about 3 to 6 bpm). Some patients may have larger increases.
Monitor all EVEKEO ODT-treated patients for potential tachycardia and hypertension.
5.4 Psychiatric Adverse Reactions
Exacerbation of Pre-Existing Psychosis
CNS stimulants may exacerbate symptoms of behavior disturbance and thought disorder in patients with a pre-existing psychotic disorder.
Induction of a Manic Episode in Patients with Bipolar Disease
CNS stimulants may induce a manic or mixed episode in patients with bipolar disorder. Prior to initiating EVEKEO ODT treatment, screen patients for risk factors for developing a manic episode (e.g., comorbid or has a history of depressive symptoms or a family history of suicide, bipolar disorder, and depression).
New Psychotic or Manic Symptoms
CNS stimulants, at the recommended dosage, may cause psychotic or manic symptoms (e.g., hallucinations, delusional thinking, or mania) in patients without prior history of psychotic illness or mania. In a pooled analysis of multiple short-term, placebo-controlled studies of CNS stimulants, psychotic or manic symptoms occurred in approximately 0.1% of CNS stimulant-treated patients compared to 0% of placebo-treated patients. If such symptoms occur, consider discontinuing EVEKEO ODT.
5.5 Long-Term Suppression of Growth in Pediatric Patients
CNS stimulants have been associated with weight loss and slowing of growth rate in pediatric patients. Closely monitor growth (weight and height) in EVEKEO ODT-treated pediatric patients treated with CNS stimulants.
Pediatric patients who are not growing or gaining height or weight as expected may need to have their treatment interrupted.
5.6 Seizures
There is some clinical evidence that stimulants may lower the convulsive threshold in patients with prior history of seizures, in patients with prior EEG abnormalities in absence of seizures, and, very rarely, in patients without a history of seizures and no prior EEG evidence of seizures. In the presence of seizures, discontinue EVEKEO ODT.
5.7 Peripheral Vasculopathy, including Raynaud's Phenomenon
CNS stimulants, including EVEKEO ODT, used to treat ADHD are associated with peripheral vasculopathy, including Raynaud's phenomenon. Signs and symptoms are usually intermittent and mild; however, sequelae have included digital ulceration and/or soft tissue breakdown. Effects of peripheral vasculopathy, including Raynaud's phenomenon, were observed in post-marketing reports and at the therapeutic dosage of CNS stimulants in all age groups throughout the course of treatment. Signs and symptoms generally improved after dosage reduction or discontinuation of the CNS stimulant.
Careful observation for digital changes is necessary during EVEKEO ODT treatment. Further clinical evaluation (e.g., rheumatology referral) may be appropriate for EVEKEO ODT-treated patients who develop signs or symptoms of peripheral vasculopathy.
5.8 Serotonin Syndrome
Serotonin syndrome, a potentially life-threatening reaction, may occur when amphetamines are used in combination with other drugs that affect the serotonergic neurotransmitter systems such as MAOIs, selective serotonin reuptake inhibitors (SSRIs), serotonin norepinephrine reuptake inhibitors (SNRIs), triptans, tricyclic antidepressants, fentanyl, lithium, tramadol, tryptophan, buspirone, and St. John's Wort [see Drug Interactions (7.1)] . The co-administration with cytochrome P450 2D6 (CYP2D6) inhibitors may also increase the risk with increased exposure to EVEKEO ODT. In these situations, consider an alternative non-serotonergic drug or an alternative drug that does not inhibit CYP2D6 [see Drug Interactions (7.1)] .
Serotonin syndrome symptoms may include mental status changes (e.g., agitation, hallucinations, delirium, and coma), autonomic instability (e.g., tachycardia, labile blood pressure, dizziness, diaphoresis, flushing, hyperthermia), neuromuscular symptoms (e.g., tremor, rigidity, myoclonus, hyperreflexia, incoordination), seizures, and/or gastrointestinal symptoms (e.g., nausea, vomiting, diarrhea).
Concomitant use of EVEKEO ODT with MAOI drugs is contraindicated [see Contraindications (4)] .
Discontinue treatment with EVEKEO ODT and any concomitant serotonergic agents immediately if the above symptoms occur, and initiate supportive symptomatic treatment. If concomitant use of EVEKEO ODT with other serotonergic drugs or CYP2D6 inhibitors is clinically warranted, initiate EVEKEO ODT with lower doses, monitor patients for the emergence of serotonin syndrome during drug initiation or titration, and inform patients of the increased risk for serotonin syndrome.
5.9 Motor and Verbal Tics, and Worsening of Tourette's Syndrome
CNS stimulants, including amphetamine sulfate, have been associated with the onset or exacerbation of motor and verbal tics. Worsening of Tourette's syndrome has also been reported [see Adverse Reactions (6.2)] .
Before initiating EVEKEO ODT, assess the family history and clinically evaluate patients for tics or Tourette's syndrome. Regularly monitor EVEKEO ODT-treated patients for the emergence or worsening of tics or Tourette's syndrome, and discontinue treatment if clinically appropriate.
6 ADVERSE REACTIONS
The following adverse reactions are discussed in greater detail in other sections of the labeling:
- Abuse, Misuse, and Addiction [see Boxed Warning, Warnings and Precautions (5.1), and Drug Abuse and Dependence (9.2, 9.3)]
- Hypersensitivity to amphetamine, or other components of EVEKEO ODT [see Contraindications (4)]
- Hypertensive Crisis When Used Concomitantly with Monoamine Oxidase Inhibitors [see Contraindications (4)and Drug Interactions (7.1)]
- Risks to Patients with Serious Cardiac Disease [see Warnings and Precautions (5.2)]
- Increased Blood Pressure and Heart Rate [see Warnings and Precautions (5.3)]
- Psychiatric Adverse Reactions [see Warnings and Precautions (5.4)]
- Seizures [see Warnings and Precautions (5.6)]
- Long-Term Suppression of Growth in Pediatric Patients [see Warnings and Precautions (5.5)]
- Peripheral Vasculopathy, including Raynaud's Phenomenon [see Warnings and Precautions (5.7)]
- Serotonin Syndrome [see Warnings and Precautions (5.8)]
- Motor and Verbal Tics, and Worsening of Tourette's Syndrome [see Warnings and Precautions (5.9)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
Study 1 was conducted with EVEKEO tablets (i.e., not the ODT formulation) in children ages 6 to 12 years who met Diagnostic and Statistical Manual of Mental Disorders, 4 thedition, Text Revision (DSM-IV-TR) criteria for ADHD. This study began with an 8-week, open-label, dose-optimization phase followed by a 2-week double-blind, placebo-controlled, randomized, crossover phase. Adverse reactions reported in > 5% of patients (N=105; doses of 10 to 40 mg/day) during the open-label phase included: decreased appetite (28%), infections (22%), abdominal pain (15%), irritability (14%), headache (13%), nausea (6%), vomiting (6%), affect lability (includes mood swings; 9%), tachycardia (9%), insomnia (10%), fatigue (10%),and dry mouth (6%). During the open-label phase, six patients discontinued due to adverse reactions: irritability (n=3), affect lability (n=1), initial insomnia (n=1), and rash (n=1).
Table 1 lists the adverse reactions reported during the double-blind, cross-over phase. No patient discontinued the study for an adverse reaction during the double-blind crossover phase. Because of the trial design (an initial 8-week, open-label, active treatment phase), the adverse reaction rates described in the double-blind phase are lower than expected in clinical practice.
| System Organ Class
Preferred Term | EVEKEO (n= 97) | Placebo (n= 97) |
|---|---|---|
| Subjects with at least one adverse event | 22% | 14% |
| Metabolism and Nutrition Disorders | ||
| Decreased appetite | 4% | 0% |
| Gastrointestinal Disorders | ||
| Abdominal pain | 3% | 0% |
| Psychiatric Disorders | ||
| Affect Lability † | 3% | 0% |
| Insomnia | 4% | 0% |
| Injury, poisoning and procedural complications | ||
| Injury | 3% | 2% |
6.2 Postmarketing Experience
The following adverse reactions have been associated during post approval use of amphetamines. Because these reactions are reported voluntarily from a population of uncertain size, it is not possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Cardiovascular:Palpitations, tachycardia, elevation of blood pressure, sudden death, myocardial infarction. There have been isolated reports of cardiomyopathy associated with chronic amphetamine use.
Central Nervous System:Psychotic episodes at recommended doses, overstimulation, irritability, restlessness, dizziness, insomnia, euphoria, mood swings, aggression, anger, logorrhea, dermatillomania, dyskinesia, dysphoria, tremor, fatigue, headache, exacerbation of motor and verbal tics and Tourette's syndrome
Gastrointestinal:Dry mouth, unpleasant taste, constipation, nausea, intestinal ischemia, other gastrointestinal disturbances, anorexia, and weight loss.
7 DRUG INTERACTIONS
7.1 Drugs Having Clinically Important Interactions with Amphetamines
| MAO Inhibitors (MAOI) | |
| Clinical Impact | MAOI antidepressants slow amphetamine metabolism, increasing amphetamines effect on the release of norepinephrine and other monoamines from adrenergic nerve endings causing headaches and other signs of hypertensive crisis. Toxic neurological effects and malignant hyperpyrexia can occur, sometimes with fatal results. |
| Intervention | Do not administer EVEKEO ODT during or within 14 days following the administration of MAOI [see Contraindications (4)] . |
| Serotonergic Drugs | |
| Clinical Impact | The concomitant use of EVEKEO ODT and serotonergic drugs increases the risk of serotonin syndrome. |
| Intervention | Initiate with lower doses and monitor patients for signs and symptoms of serotonin syndrome, particularly during EVEKEO ODT initiation or dosage increase. If serotonin syndrome occurs, discontinue EVEKEO ODT and concomitant serotonergic drug(s) [see Warnings and Precautions 5.8] . |
| Alkalinizing Agents | |
| Clinical Impact | May increase exposure to amphetamine and exacerbate the action of amphetamine. |
| Intervention | Caution should be taken when co-administering EVEKEO ODT and gastrointestinal and urinary alkalinizing agents. |
| Acidifying Agents | |
| Clinical Impact | Lower blood levels and efficacy of amphetamines. |
| Intervention | Increase dose of EVEKEO ODT based on clinical response. |
| Tricyclic Antidepressants | |
| Clinical Impact | May enhance the activity of tricyclic or sympathomimetic agents causing sustained increases in the concentration of d- amphetamine in the brain; cardiovascular effects can be potentiated. |
| Intervention | Monitor frequently and adjust EVEKEO ODT dose or use alternative therapy based on clinical response. |
| CYP2D6 Inhibitors | |
| Clinical Impact | The concomitant use of EVEKEO ODT and CYP2D6 inhibitors may increase the exposure of EVEKEO ODT compared to the use of the drug alone and increase the risk of serotonin syndrome. |
| Intervention | Initiate with lower doses and monitor patients for signs and symptoms of serotonin syndrome particularly during EVEKEO ODT initiation and after a dosage increase. If serotonin syndrome occurs, discontinue EVEKEO ODT and the CYP2D6 inhibitor. Alternatively, consider using a drug that does not inhibit CYP2D6 [see Warnings and Precautions (5.8)and Overdosage (10)] . |
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to ADHD medications, including EVEKEO ODT, during pregnancy. Healthcare providers are encouraged to register patients by calling the National Pregnancy Registry for Psychostimulants at 1-866-961-2388 or visiting online at https://womensmentalhealth.org/clinical-and-research-programs/pregnancyregistry/othermedications/.
Risk Summary
Available data from published epidemiologic studies and postmarketing reports on use of prescription amphetamine in pregnant women have not identified a drug-associated risk of major birth defects and miscarriage. Adverse pregnancy outcomes, including premature delivery and low birth weight, have been seen in infants born to mothers taking amphetamines during pregnancy (see Clinical Considerations) .
Dextroamphetamine sulfate has been shown to have embryotoxic effects when administered to A/Jax mice and C57BL mice in doses approximately 6 times the maximum human dose. Embryotoxic effects were not seen in New Zealand white rabbits.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Clinical Considerations
Fetal/Neonatal Adverse Reactions
Amphetamines, such as EVEKEO ODT, cause vasoconstriction and thereby decrease placental perfusion. In addition, amphetamines can stimulate uterine contractions, increasing the risk of premature delivery. Infants born to mothers taking amphetamines during pregnancy have an increased risk of premature delivery and low birth weight.
Monitor infants born to mothers taking amphetamines for symptoms of withdrawal such as feeding difficulties, irritability, agitation, and excessive drowsiness.
8.2 Lactation
Risk Summary
Based on limited case reports in published literature, amphetamine ( d-or dl) is present in human milk at relative infant doses of 2% to 13.8% of the maternal weight-adjusted dosage and a milk/plasma ratio ranging between 1.9 and 7.5. There are no reports of adverse effects on the breastfed infant. Long-term neurodevelopmental effects on infants from amphetamine exposure are unknown. It is possible that large dosages of amphetamine might interfere with milk production, especially in women whose lactation is not well established. Because of the potential for serious adverse reactions in nursing infants, advise patients that breast feeding is not recommended during treatment with EVEKEO ODT.
8.4 Pediatric Use
The safety and effectiveness of EVEKEO ODT have been established in pediatric patients 6 years and older. Use of EVEKEO ODT is based on one adequate and well-controlled study with another immediate-release amphetamine sulfate product (EVEKEO) in pediatric patients 6 to 12 years [see Clinical Studies (14)], along with dosing and safety information for other amphetamine products.
The safety and efficacy in pediatric patients less than 6 years have not been established.
Long-Term Growth Suppression
Growth should be monitored during treatment with stimulants, including EVEKEO ODT. Pediatric patients aged 6 to 17 years who are not growing or gaining weight as expected may need to have their treatment interrupted [see Warnings and Precautions (5.5), Adverse Reactions (6.1)] .
9 DRUG ABUSE AND DEPENDENCE
9.2 Abuse
EVEKEO ODT has a high potential for abuse and misuse which can lead to the development of a substance use disorder, including addiction [see Warnings and Precautions (5.1)] . EVEKEO ODT can be diverted for non-medical use into illicit channels or distribution.
Abuse is the intentional non-therapeutic use of a drug, even once, to achieve a desired psychological or physiological effect. Misuse is the intentional use, for therapeutic purposes, of a drug by an individual in a way other than prescribed by a health care provider or for whom it was not prescribed. Drug addiction is a cluster of behavioral, cognitive, and physiological phenomena that may include a strong desire to take the drug, difficulties in controlling drug use (e.g., continuing drug use despite harmful consequences, giving a higher priority to drug use than other activities and obligations), and possible tolerance or physical dependence.
Misuse and abuse of amphetamine may cause increased heart rate, respiratory rate, or blood pressure; sweating; dilated pupils; hyperactivity; restlessness; insomnia; decreased appetite; loss of coordination; tremors; flushed skin; vomiting; and/or abdominal pain. Anxiety, psychosis, hostility, aggression, and suicidal or homicidal ideation have also been observed with CNS stimulants abuse and/or misuse. Misuse and abuse of CNS stimulants, including EVEKEO ODT, can result in overdose and death [see Overdosage (10)] , and this risk is increased with higher doses or unapproved methods of administration, such as snorting or injection.
9.3 Dependence
Physical Dependence
EVEKEO ODT may produce physical dependence. Physical dependence is a state that develops as a result of physiological adaptation in response to repeated drug use, manifested by withdrawal signs and symptoms after abrupt discontinuation or a significant dose reduction of a drug.
Withdrawal signs and symptoms after abrupt discontinuation or dose reduction following prolonged use of CNS stimulants including EVEKEO ODT include dysphoric mood; depression; fatigue; vivid, unpleasant dreams; insomnia or hypersomnia; increased appetite; and psychomotor retardation or agitation.
10 OVERDOSAGE
Clinical Effects of Overdose
Overdose of CNS stimulants is characterized by the following sympathomimetic effects:
- Cardiovascular effects including tachyarrhythmias, and hypertension or hypotension. Vasospasm, myocardial infarction, or aortic dissection may precipitate sudden cardiac death. Takotsubo cardiomyopathy may develop.
- CNS effects including psychomotor agitation, confusion, and hallucinations. Serotonin syndrome, seizures, cerebral vascular accidents, and coma may occur.
- Life-threatening hyperthermia (temperatures greater than 104°F) and rhabdomyolysis may develop.
11 DESCRIPTION
EVEKEO ODT orally disintegrating tablets contain amphetamine sulfate, a CNS stimulant, as a 1 to 1 ratio of dextroamphetamine sulfate and levoamphetamine sulfate (d- and l-amphetamine sulfate). Amphetamine sulfate is a white, odorless crystalline powder. It has a slightly bitter taste. Its solutions are acid to litmus, having a pH of 5.0 to 6.0. It is freely soluble in water and slightly soluble in alcohol.
Structural Formula:
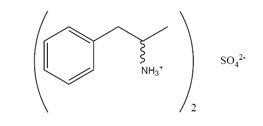
EVEKEO ODT tablets are intended for oral use. Each EVEKEO ODT tablet contains 5 mg, 10 mg, 15 mg, or 20 mg of racemic amphetamine sulfate. Each tablet also contains the following inactive ingredients: amino methacrylate copolymer, citric acid, crospovidone, ethylcellulose, dibutyl sebacate, magnesium stearate, malic acid, mannitol, microcrystalline cellulose, and sucralose.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Amphetamines are non-catecholamine sympathomimetic amines with central nervous system (CNS) stimulant activity. The mode of therapeutic action in ADHD is not known.
12.2 Pharmacodynamics
Amphetamines block the reuptake of norepinephrine and dopamine into the presynaptic neuron and increase the release of these monoamines into the extraneuronal space.
EVEKEO ODT is a 1:1 racemic mixture of d- and l-amphetamine. The l-isomer is more potent than the d-isomer in cardiovascular activity while the d-isomer is more potent than the l-isomer in causing CNS excitatory effects.
12.3 Pharmacokinetics
Amphetamine demonstrates linear pharmacokinetics over the dose range of 5 to 40 mg.
Absorption
Following a single-dose oral administration of Evekeo ODT 20 mg disintegrated/dissolved in the oral cavity in healthy subjects in a crossover study, exposures (C maxand AUC) to d- and l-amphetamine were comparable to that after administration of equal dose of immediate-release amphetamine sulfate tablets (Evekeo) tablets swallowed intact with water.
Median (range) T maxof d-and l-amphetamine was reached at approximately 3.5 (2-8) hours and 3.0 (1 -6) hours after administration without water and with water, respectively.
Effect of Food
Administration of food (a high fat meal) does not affect the observed AUC and C maxof d-and l-amphetamine after single-dose oral administration of EVEKEO ODT (20 mg) in healthy adults who allowed the tablet to be disintegrated/dissolved in their oral cavity prior to swallowing without water. Median (range) T maxincreased from 2.5 (1.5 – 6) hours to 4.5 (2.5 – 8.0) hours when administration without compared to with food.
Elimination
Amphetamine undergoes both hepatic and renal elimination. The plasma elimination half-life of d-and l-amphetamine averaged 10.0 and about 11.7 hours in healthy adult volunteers.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
No evidence of carcinogenicity was found in studies in which d-, l-amphetamine (enantiomer ratio of 1:1) was administered to mice and rats in the diet for 2 years at doses of up to 30 mg/kg/day in male mice, 19 mg/kg/day in female mice, and 5 mg/kg/day in male and female rats. These doses are approximately 4, 2, and 1 times, respectively, the maximum recommended human dose of 40 mg/day, on a mg/m 2basis.
14 CLINICAL STUDIES
The safety and effectiveness of EVEKEO ODT for the treatment of ADHD has been established based on an adequate and well-controlled study of immediate-release amphetamine sulfate (EVEKEO). Below is a description of this study and its results.
Study 1 (NCT01986062) was conducted with EVEKEO tablets in children ages 6 to 12 years who met DSM-IV-TR criteria for ADHD. Following 8 weeks of open-label dose optimization, patients were randomly assigned to continue their optimized dose of EVEKEO (10 to 40 mg/day in divided doses) or placebo for 1 week. After 1 week, patients crossed-over to receive the alternate treatment. At the end of each treatment week, efficacy assessments were conducted at 0.75, 2, 4, 6, 8, and 10 hours post-dose using the Swanson, Kotkin, Agler, M-Flynn, and Pelham (SKAMP) rating scale. SKAMP is a 13-item teacher-rated scale that assesses manifestations of ADHD in a classroom setting. The SKAMP-Combined score was obtained by summing items 1 through 13. The primary efficacy outcome assessed by the SKAMP-Combined score at 2 hours postdose was statistically significantly better in EVEKEO treatment compared to placebo (Table 3). Key secondary efficacy endpoints were the time-to-onset and duration-of-effect of EVEKEO using SKAMP-Combined scores. SKAMP-Combined scores were statistically significantly better for patients in the EVEKEO treatment group compared to patients in the placebo treatment group beginning at 0.75 hours post-dose and at each assessment through 10 hours post-dose. (Figure 1).
| Study Number | Treatment Group | Primary Efficacy Measure: SKAMP-Combined Score at 2 Hours Post-dose | ||
|---|---|---|---|---|
| Mean Pre-Dose Score (SD) | LS Mean (SE) at 2 Hours Post-dose | Placebo-subtracted Difference *(95% CI) | ||
| SD: standard deviation; SE: standard error; LS Mean: least-squares mean; CI: confidence interval. | ||||
|
||||
| Study 1 | Evekeo | 18.1 (11.6) | 10.3 (1.09) | -7.9 (-10.1, -5.6) |
| Placebo | 15.3 (11.4) | 18.1 (1.09) | -- | |
Figure 1: LS Mean SKAMP-Combined Scores by Treatment and Timepoint for Pediatric Patients (6 to 12 years) with ADHD after 1 Week of Double-Blind Treatment (Study 1)
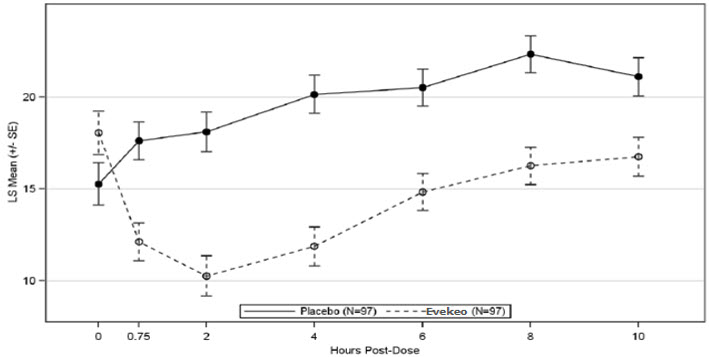
The values at pre-dose hour (zero) are observed means.
16 HOW SUPPLIED/STORAGE AND HANDLING
EVEKEO ODT (amphetamine sulfate) orally disintegrating tablets are supplied as follows:
- 5 mg: white to off-white, round, flat-faced radius-edged tablet with "5" on one side and "EVI" on the other
- NDC 24338-031-30: One blister card of 30-count 5 mg strength tablets within a plastic sleeve
- NDC 24338-031-01: Carton containing one plastic sleeve.
- 10 mg: white to off-white, round, flat-faced radius-edged tablet with "10" on one side and "EVI" on the other
- NDC 24338-033-30: One blister card of 30-count 10 mg strength tablets within a plastic sleeve
- NDC 24338-033-01: Carton containing one plastic sleeve
- 15 mg: white to off-white, round, flat-faced radius-edged tablet with "15" on one side and "EVI" on the other
- NDC 24338-035-30: One blister card of 30-count 15 mg strength tablets within a plastic sleeve
- NDC 24338-035-01: Carton containing one plastic sleeve
- 20 mg: white to off-white, round, flat-faced radius-edged tablet with "20" on one side and "EVI" on the other
- NDC 24338-037-15: One blister card of 15-count 20 mg strength tablets within a plastic sleeve
- NDC 24338-037-02: Carton containing two 15-count plastic sleeves
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Abuse, Misuse, and Addiction
Educate patients and their families about the risks of abuse, misuse, and addiction of EVEKEO ODT, which can lead to overdose and death, and proper disposal of any unused drug [see Warnings and Precautions (5.1), Drug Abuse and Dependence (9.2), Overdosage (10)]. Advise patients to store EVEKEO ODT in a safe place, preferably locked, and instruct patients to not give EVEKEO ODT to anyone else.
Dosage and Administration Instructions
Provide the following instructions on administration to the patient:
- The tablet should remain in the blister pack until the patient is ready to take it.
- The patient or caregiver should use dry hands to open the blister.
- Remove the tablet by pushing it through the back of the foil-lined blister packaging.
- As soon as the blister is opened, place the tablet on the patient's tongue.
- The whole tablet should be placed on the tongue and allowed to disintegrate without chewing or crushing.
- The tablet will disintegrate in saliva so that it can be swallowed.
Risks to Patients with Serious Cardiac Disease
Advise patients that there are potential risks to patients with serious cardiac disease, including sudden death, with EVEKEO ODT use. Instruct patients to contact a healthcare provider immediately if they develop symptoms such as exertional chest pain, unexplained syncope, or other symptoms suggestive of cardiac disease [see Warnings and Precautions (5.2)] .
Increased Blood Pressure and Heart Rate
Instruct patients and their caregivers that EVEKEO ODT can cause elevations of their blood pressure and pulse rate and that patients should be monitored for such effects [see Warnings and Precautions (5.3)] .
Psychiatric Adverse Reactions
Advise patients and their caregivers that EVEKEO ODT, at recommended doses, may cause psychotic symptoms or mania even in patients without prior history of psychotic symptoms or mania [see Warnings and Precautions (5.4)] .
Long-Term Suppression of Growth in Pediatric Patients
Advise patients, family members, and caregivers that EVEKEO ODT may cause slowing of growth including weight loss [see Warnings and Precautions (5.5)] .
Circulation Problems in Fingers and Toes [Peripheral Vasculopathy, including Raynaud's Phenomenon]
Instruct patients and their caregivers beginning treatment with EVEKEO ODT about the risk of peripheral vasculopathy, including Raynaud's phenomenon, and associated signs and symptoms: fingers or toes may feel numb, cool, painful, and/or may change from pale, to blue, to red. Instruct patients to report to their physician any new numbness, pain, skin color change, or sensitivity to temperature in fingers or toes. Instruct patients to call their physician immediately with any signs of unexplained wounds appearing on fingers or toes while taking EVEKEO ODT. Further clinical evaluation (e.g. rheumatology referral) may be appropriate for certain patients [see Warnings and Precautions (5.7)] .
Serotonin Syndrome
Caution patients and their caregivers about the risk of serotonin syndrome with concomitant use of EVEKEO ODT and other serotonergic drugs including SSRIs, SNRIs, triptans, tricyclic antidepressants, fentanyl, lithium, tramadol, tryptophan, buspirone, St. John's Wort, and with drugs that impair metabolism of serotonin (in particular MAOIs, both those intended to treat psychiatric disorders and also others such as linezolid [see Contraindications (4), Warnings and Precautions (5.8)and Drug Interactions (7.1)]. Advise patients to contact their healthcare provider or report to the emergency room if they experience signs or symptoms of serotonin syndrome.
Motor and Verbal Tics, and Worsening of Tourette's Syndrome
Advise patients that motor and verbal tics and worsening of Tourette's Syndrome may occur during treatment with EVEKEO ODT. Instruct patients to notify their healthcare provider if emergence of new tics or worsening of tics or Tourette's syndrome occurs [see Warnings and Precautions (5.9)] .
Concomitant Medications
Advise patients and their caregivers to notify their physicians if they are taking, or plan to take, any prescription or over-the-counter drugs because there is a potential for interactions [see Drug Interactions (7.1)] .
Pregnancy Registry
Advise patients that there is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to EVEKEO ODT during pregnancy [see Use in Specific Populations (8.1)].
Pregnancy
Advise patients to notify their healthcare provider if they become pregnant or intend to become pregnant during treatment with EVEKEO ODT [see Use in Specific Populations (8.1)] . Advise patients of the potential fetal effects from the use of EVEKEO ODT during pregnancy [see Use in Specific Populations (8.1)].
Lactation
Advise patients not to breastfeed if they are taking EVEKEO ODT [see Use in Specific Populations (8.2)] .
Evekeo ODT® manufactured for Arbor Pharmaceuticals, LLC Atlanta, GA 30328 by Adare Pharmaceuticals, Inc. Vandalia, OH 45377
| MEDICATION GUIDE
EVEKEO ODT ®(ee-VEEK-ee-o) (amphetamine sulfate) orally disintegrating tablets, CII |
||
|---|---|---|
| This Medication Guide has been approved by the U.S. Food and Drug Administration. Revised: 10/2023 | ||
| EVK-MG-04 | ||
| What is the most important information I should know about EVEKEO ODT? | ||
EVEKEO ODT may cause serious side effects, including:
|
||
| What is EVEKEO ODT? | ||
| EVEKEO ODT is a central nervous system (CNS) stimulant prescription medicine used for the treatment of Attention Deficit Hyperactivity Disorder (ADHD) in children 6 to 17 years of age. EVEKEO ODT may help increase attention and decrease impulsiveness and hyperactivity in people with ADHD. | ||
| It is not known if EVEKEO ODT is safe and effective in children under 6 years of age. | ||
| EVEKEO ODT is a federally controlled substance (CII) because it contains amphetamine that can be a target for people who abuse prescription medicines or street drugs.Keep EVEKEO ODT in a safe place to protect it from theft. Never give your EVEKEO ODT to anyone else, because it may cause death or harm them. Selling or giving away EVEKEO ODT may harm others and is against the law. | ||
Do not take EVEKEO ODT if you or your child are:
|
||
Before taking EVEKEO ODT, tell your healthcare provider about all medical conditions, including if you or your child:
|
||
| Tell your healthcare provider about all the medicines that you or your child take,including prescription and over-the-counter medicines, vitamins, and herbal supplements. | ||
| EVEKEO ODT and some medicines may interact with each other and cause serious side effects. Sometimes the doses of other medicines will need to be changed while taking EVEKEO ODT. | ||
| Especially tell your healthcare provider if you or your child takemedicines used to treat depression including MAOIs. | ||
| Know the medicines that you or your child takes. Keep a list of all medicines with you to show your healthcare provider and pharmacist when you get a new medicine. | ||
| Your healthcare provider will decide whether EVEKEO ODT can be taken with other medicines. Do not start any new medicine during treatment with EVEKEO ODT without talking to your healthcare provider first. | ||
How should EVEKEO ODT be taken?
|
||
Use the following instructions when taking EVEKEO ODT:
|
||
| If you or your child take too much EVEKEO ODT, call your healthcare provider or Poison Help line at 1-800-222-1222 or go to the nearest hospital emergency room right away. | ||
| What are possible side effects of EVEKEO ODT? | ||
EVEKEO ODT may cause serious side effects, including:
|
||
|
|
|
| The most common side effects of EVEKEO ODT includedecreased appetite and trouble sleeping. | ||
| These are not all the possible side effects of EVEKEO ODT. | ||
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
|
||
How should I store EVEKEO ODT?
|
||
| Keep EVEKEO ODT and all medicines out of the reach of children. | ||
| General information about the safe and effective use of EVEKEO ODT. | ||
| Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use EVEKEO ODT for a condition for which it was not prescribed. Do not give EVEKEO ODT to other people, even if they have the same symptoms. It may harm them and it is against the law. You can ask your healthcare provider or pharmacist for information about EVEKEO ODT that is written for healthcare professionals. | ||
| What are the ingredients in EVEKEO ODT? | ||
| Active ingredient:amphetamine sulfate | ||
| Inactive ingredients:mannitol, silicified microcrystalline cellulose, crospovidone, ethylcellulose, amino methacrylate copolymer, anhydrous citric acid, magnesium stearate, dibutyl sebacate, malic acid and sucralose | ||
| Distributed by: Arbor ®Pharmaceuticals, LLC, Atlanta, GA 30328 | ||
| All rights reserved. | ||
| For more information about EVEKEO ODT, please contact Arbor Pharmaceuticals, LLC at 1-800-461-7449 | ||
PRINCIPAL DISPLAY PANEL - 5 mg Tablet Blister Pack Case Label
NDC 24338-031-30
Rx Only
EVEKEO ODT™ CII
amphetamine sulfate
ORALLY DISINTEGRATING TABLET
5 mg tablets
Do not crush or chew tablets.
Dispenser: Please provide the accompanying
Medication Guide to each patient.
Contains 30 Tablets
arbor
®
PHARMACEUTICALS, LLC

PRINCIPAL DISPLAY PANEL - 10 mg Tablet Blister Pack Case Label
NDC 24338-033-30
Rx Only
EVEKEO ODT™ CII
amphetamine sulfate
ORALLY DISINTEGRATING TABLET
10 mg tablets
Do not crush or chew tablets.
Dispenser: Please provide the accompanying
Medication Guide to each patient.
Contains 30 Tablets
arbor
®
PHARMACEUTICALS, LLC

PRINCIPAL DISPLAY PANEL - 15 mg Tablet Blister Pack Case Label
NDC 24338-035-30
Rx Only
EVEKEO ODT™ CII
amphetamine sulfate
ORALLY DISINTEGRATING TABLET
15 mg tablets
Do not crush or chew tablets.
Dispenser: Please provide the accompanying
Medication Guide to each patient.
Contains 30 Tablets
arbor
®
PHARMACEUTICALS, LLC
PRINCIPAL DISPLAY PANEL - 20 mg Tablet Blister Pack Case Label
NDC 24338-037-15
Rx Only
EVEKEO ODT™ CII
amphetamine sulfate
ORALLY DISINTEGRATING TABLET
20 mg tablets
Do not crush or chew tablets.
Dispenser: Please provide the accompanying
Medication Guide to each patient.
Contains 15 Tablets
arbor
®
PHARMACEUTICALS, LLC
| EVEKEO ODT
amphetamine sulfate tablet, orally disintegrating |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| EVEKEO ODT
amphetamine sulfate tablet, orally disintegrating |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| EVEKEO ODT
amphetamine sulfate tablet, orally disintegrating |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| EVEKEO ODT
amphetamine sulfate tablet, orally disintegrating |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - Azurity Pharmaceuticals, Inc. (117505635) |