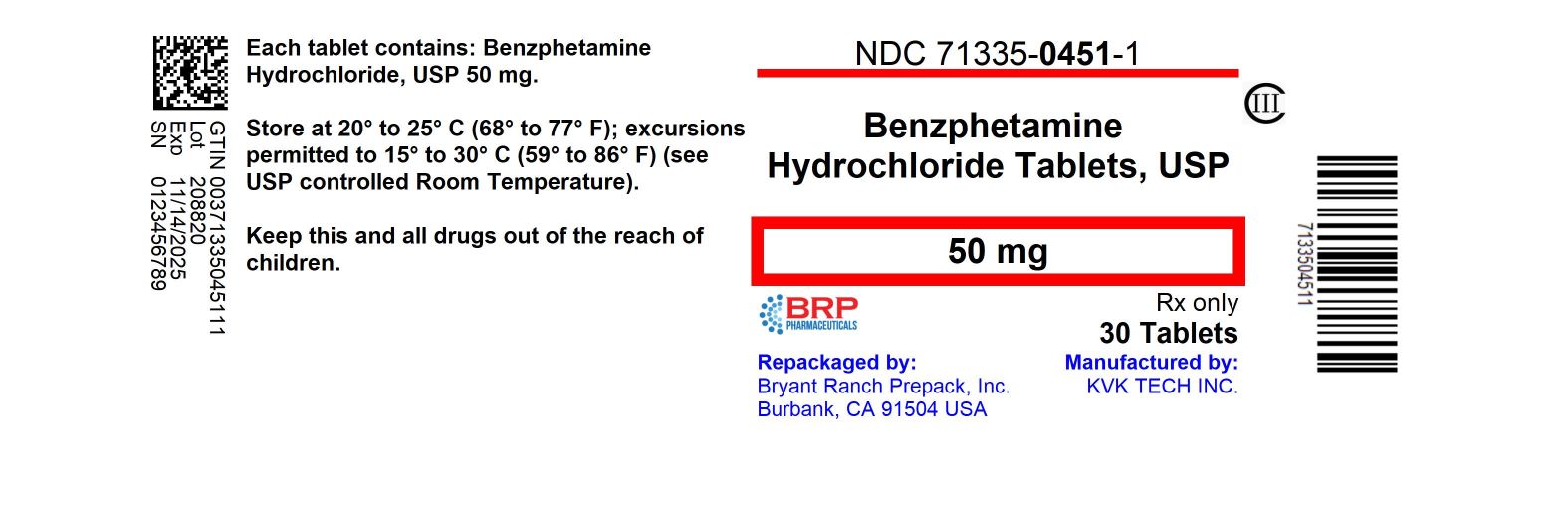
Label: BENZPHETAMINE HYDROCHLORIDE tablet
-
NDC Code(s):
71335-0451-1,
71335-0451-2,
71335-0451-3,
71335-0451-4, view more71335-0451-5, 71335-0451-6, 71335-0451-7, 71335-0451-8, 71335-0451-9
- Packager: Bryant Ranch Prepack
- This is a repackaged label.
- Source NDC Code(s): 10702-040
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: CIII
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated November 14, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
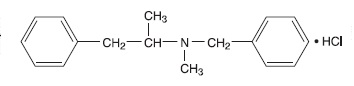
Benzphetamine hydrochloride tablets contain the anorectic agent benzphetamine hydrochloride. Benzphetamine hydrochloride is a white crystalline powder readily soluble in water and 95% ethanol. The chemical name for benzphetamine hydrochloride is d-N,α-Dimethyl-N -(phenylmethyl)-benzeneethanamine hydrochloride and its molecular weight is 275.82.
The structural formula (dextro form) is represented below:
Each benzphetamine hydrochloride tablet, for oral administration, contains 50 mg of benzphetamine hydrochloride.
Inactive Ingredients: carnauba wax powder, colloidal silicon dioxide, FD&C red # 40 aluminum lake, FD&C yellow # 6 aluminum lake, lactose monohydrate, macrogol/polyethylene glycol 3350, magnesium stearate, microcrystalline cellulose 101, polyvinyl alcohol – partially hydrolyzed, sodium starch glycolate, talc and titanium dioxide.
-
CLINICAL PHARMACOLOGY
Benzphetamine hydrochloride is a sympathomimetic amine with pharmacologic activity similar to the prototype drugs of this class used in obesity, the amphetamines. Actions include central nervous system stimulation and elevation of blood pressure. Tachyphylaxis and tolerance have been demonstrated with all drugs of this class in which these phenomena have been looked for.
Drugs of this class used in obesity are commonly known as "anorectics" or "anorexigenics". It has not been established, however, that the action of such drugs in treating obesity is primarily one of appetite suppression. Other central nervous system actions, or metabolic effects, may be involved.
Adult obese subjects instructed in dietary management and treated with "anorectic" drugs, lose more weight on the average than those treated with placebo and diet, as determined in relatively short-term clinical trials.
The magnitude of increased weight loss of drug-treated patients over placebo-treated patients is only a fraction of a pound a week. The rate of weight loss is the greatest in the first weeks of therapy for both drug and placebo subjects and tends to decrease in succeeding weeks. The possible origins of the increased weight loss due to the various drug effects are not established. The amount of weight loss associated with the use of an "anorectic" drug varies from trial to trial, and the increased weight loss appears to be related in part to variables other than the drug prescribed, such as the physician-investigator, the population treated, and the diet prescribed. Studies do not permit conclusions as to the relative importance of the drug and non-drug factors on weight loss.
The natural history of obesity is measured in years, whereas the studies cited are restricted to a few weeks duration; thus, the total impact of drug-induced weight loss over that of diet alone must be considered to be clinically limited.
Pharmacokinetic data in humans are not available.
-
INDICATIONS AND USAGE
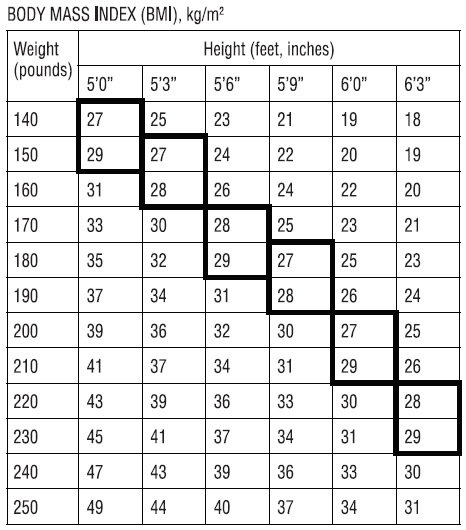
Benzphetamine hydrochloride tablets, USP are indicated in the management of exogenous obesity as a short term (a few weeks) adjunct in a regimen of weight reduction based on caloric restriction in patients with an initial body mass index (BMI) of 30 kg/m2 or higher who have not responded to appropriate weight reducing regimen (diet and/or exercise) alone. Below is a chart of Body Mass Index (BMI) based on various heights and weights. BMI is calculated by taking the patient's weight, in kilograms (kg), divided by the patient's height, in meters (m), squared. Metric conversions are as follows: pounds ÷2.2 = kg; inches × 0.0254 = meters. The limited usefulness of agents of this class (See CLINICAL PHARMACOLOGY) should be weighed against possible risks inherent in their use such as those described below.
Benzphetamine hydrochloride tablets, USP are indicated for use as monotherapy only.
-
CONTRAINDICATIONS
Benzphetamine hydrochloride tablets, USP are contraindicated in patients with advanced arteriosclerosis, symptomatic cardiovascular disease, moderate to severe hypertension, hyperthyroidism, known hypersensitivity or idiosyncrasy to sympathomimetic amines, and glaucoma. Benzphetamine should not be given to patients who are in an agitated state or who have a history of drug abuse.
Hypertensive crises have resulted when sympathomimetic amines have been used concomitantly or within 14 days following use of monoamine oxidase inhibitors. Benzphetamine hydrochloride tablets should not be used concomitantly with other CNS stimulants.
Benzphetamine hydrochloride tablets may cause fetal harm when administered to a pregnant woman. Amphetamines have been shown to be teratogenic and embryotoxic in mammals at high multiples of the human dose. Benzphetamine hydrochloride tablets are contraindicated in women who are or may become pregnant. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus.
-
WARNINGS
Benzphetamine hydrochloride tablets, USP should not be used in combination with other anorectic agents, including prescribed drugs, over-the-counter preparations and herbal products.
In a case-control epidemiological study, the use of anorectic agents was associated with an increased risk of developing pulmonary hypertension, a rare, but often fatal disorder. The use of anorectic agents for longer than three months was associated with a 23-fold increase in the risk of developing pulmonary hypertension. Increased risk of pulmonary hypertension with repeated courses of therapy cannot be excluded. It should be noted that benzphetamine was not specifically studied in this case-control study.
The onset or aggravation of exertional dyspnea, or unexplained symptoms of angina pectoris, syncope, or lower extremity edema suggest the possibility of occurrence of pulmonary hypertension. Under these circumstances, Benzphetamine hydrochloride tablets, USP should be immediately discontinued, and the patient should be evaluated for the possible presence of pulmonary hypertension.
Valvular heart disease associated with the use of some anorectic agents such as fenfluramine and dexfenfluramine has been reported. Possible contributing factors include use for extended periods of time, higher than recommended dose, and/or use in combination with other anorectic drugs. However, no cases of this valvulopathy have been reported when benzphetamine has been used alone.
The potential risk of possible serious adverse effects such as valvular heart disease and pulmonary hypertension should be assessed carefully against the potential benefit of weight loss. Baseline cardiac evaluation should be considered to detect pre-existing valvular heart diseases or pulmonary hypertension prior to initiation of benzphetamine treatment. Benzphetamine hydrochloride tablets, USP are not recommended in patients with known heart murmur or valvular heart disease. Echocardiogram during and after treatment could be useful for detecting any valvular disorders which may occur. To limit unwarranted exposure and risks, treatment with benzphetamine hydrochloride tablets, USP should be continued only if the patient has satisfactory weight loss within the first 4 weeks of treatment (i.e., weight loss of at least 4 pounds, or as determined by the physician and patient).
When tolerance to the anorectic effect develops, the recommended dose should not be exceeded in an attempt to increase the effect; rather, the drug should be discontinued.
Benzphetamine hydrochloride tablets, USP are not recommended for severely hypertensive patients or for patients with symptomatic cardiovascular disease including arrhythmias.
Benzphetamine hydrochloride tablets, USP are not recommended for patients who used any anorectic agents within the prior year.
-
PRECAUTIONS
General
Insulin requirements in diabetes mellitus may be altered in association with use of anorexigenic drugs and the concomitant dietary restrictions.
Psychological disturbances have been reported in patients who receive an anorectic agent together with a restrictive dietary regime.
Caution is to be exercised in prescribing amphetamines for patients with even mild hypertension. The least amount feasible should be prescribed or dispensed at one time in order to minimize the possibility of overdosage.
Information for Patients
Amphetamines may impair the ability of the patient to engage in potentially hazardous activities such as operating machinery or driving a motor vehicle; the patient should therefore be cautioned accordingly.
Drug Interactions
Efficacy of benzphetamine hydrochloride tablets, USP in combination with other anorectic agents has not been studied and the combined use may have the potential for serious cardiac problems.
Hypertensive crises have resulted when sympathomimetic amines have been used concomitantly or within 14 days following use of monoamine oxidase inhibitors. Benzphetamine hydrochloride tablets, USP should not be used concomitantly with other CNS stimulants.
Amphetamines may decrease the hypotensive effect of antihypertensives. Amphetamines may enhance the effects of tricyclic antidepressants.
Urinary alkalinizing agents increase blood levels and decrease excretion of amphetamines. Urinary acidifying agents decrease blood levels and increase excretion of amphetamines.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Animal studies to evaluate the potential for carcinogenesis, mutagenesis or impairment of fertility have not been performed by Pharmacia & Upjohn Company.
Nursing Mothers
Amphetamines are excreted in human milk. Mothers taking amphetamines should be advised to refrain from nursing.
Pediatric Use
Safety and effectiveness in pediatric patients have not been established. The use of benzphetamine hydrochloride tablets, USP are not recommended in patients less than 17 years of age.
Geriatric Use
Clinical studies of benzphetamine hydrochloride tablets, USP did not include sufficient numbers of subjects aged 65 and over to establish safety and efficacy in this population. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
-
ADVERSE REACTIONS
The following have been associated with the use of benzphetamine hydrochloride:
Cardiovascular
Palpitation, tachycardia, elevation of blood pressure.
There have been isolated reports of cardiomyopathy and ischemic cardiac events associated with chronic amphetamine use.
Valvular heart disease associated with the use of some anorectic agents such as fenfluramine and dexfenfluramine, both independently and especially when used in combination with other anorectic drugs, have been reported. However, no cases of this valvulopathy have been reported when benzphetamine hydrochloride tablets, USP have been used alone.
CNS
Overstimulation, restlessness, dizziness, insomnia, tremor, sweating, headache; rarely, psychotic episodes at recommended doses; depression following withdrawal of the drug.
-
DRUG ABUSE AND DEPENDENCE
Benzphetamine is a controlled substance under the Controlled Substance Act by the Drug Enforcement Administration and has been assigned to Schedule III.
Benzphetamine hydrochloride is related chemically and pharmacologically to the amphetamines. Amphetamines and related stimulant drugs have been extensively abused, and the possibility of abuse of benzphetamine hydrochloride tablets, USP should be kept in mind when evaluating the desirability of including a drug as part of a weight reduction program. Abuse of amphetamines and related drugs may be associated with intense psychological dependence and severe social dysfunction. There are reports of patients who have increased the dosage to many times that recommended. Abrupt cessation following prolonged high dosage administration results in extreme fatigue and mental depression; changes are also noted on the sleep EEG. Manifestations of chronic intoxication with anorectic drugs include severe dermatoses, marked insomnia, irritability, hyperactivity, and personality changes. The most severe manifestation of chronic intoxication is psychosis, often clinically indistinguishable from schizophrenia.
-
OVERDOSAGE
Manifestations of Overdosage
Acute overdosage with amphetamines may result in restlessness, tremor, tachypnea, confusion, assaultiveness and panic states. Fatigue and depression usually follow the central stimulation. Cardiovascular effects include arrhythmias, hypertension or hypotension, and circulatory collapse. Gastrointestinal symptoms include nausea, vomiting, diarrhea, and abdominal cramps. Hyperpyrexia and rhabdomyolysis have been reported and can lead to a number of associated complications. Fatal poisoning is usually preceded by convulsions and coma.
Treatment of Overdosage
(See WARNINGS)--- Information concerning the effects of overdosage with benzphetamine hydrochloride tablets, USP is extremely limited. The following is based on experience with other anorexiants.
Management of acute amphetamine intoxication is largely symptomatic and includes sedation with a barbiturate. If hypertension is marked, the use of a nitrite or rapidly acting alpha receptor blocking agent should be considered. Experience with hemodialysis or peritoneal dialysis is inadequate to permit recommendations in this regard.
Acidification of the urine increases amphetamine excretion.
The oral LD 50 is 174 mg/kg in mice and 104 mg/kg in rats. The intraperitoneal LD 50 in mice is 153 mg/kg.
-
DOSAGE AND ADMINISTRATION
Dosage should be individualized according to the response of the patient. The suggested dosage ranges from 25 to 50 mg one to three times daily. Treatment should begin with 25 to 50 mg once daily with subsequent increase in individual dose or frequency according to response. A single daily dose is preferably given in mid-morning or mid-afternoon, according to the patient’s eating habits. In an occasional patient it may be desirable to avoid late afternoon administration. Use of benzphetamine hydrochloride is not recommended in individuals under 17 years of age.
-
HOW SUPPLIED
Benzphetamine hydrochloride tablets USP, 50 mg are supplied as peach, round, biconvex, film coated tablets debossed with “K” on left of the score and the number “40” debossed right of the score and plain on other side.
NDC: 71335-0451-1: 30 Tablets in a BOTTLE
NDC: 71335-0451-2: 7 Tablets in a BOTTLE
NDC: 71335-0451-3: 14 Tablets in a BOTTLE
NDC: 71335-0451-4: 21 Tablets in a BOTTLE
NDC: 71335-0451-5: 42 Tablets in a BOTTLE
NDC: 71335-0451-6: 84 Tablets in a BOTTLE
NDC: 71335-0451-7: 60 Tablets in a BOTTLE
NDC: 71335-0451-8: 90 Tablets in a BOTTLE
NDC: 71335-0451-9: 120 Tablets in a BOTTLE
Dispense in a tight container as defined in the USP, with a child-resistant closure (as required).
Store at 20° to 25°C with excursions permitted between 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature].
Rx only
Repackaged/Relabeled by:
Bryant Ranch Prepack, Inc.
Burbank, CA 9150 - PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
BENZPHETAMINE HYDROCHLORIDE
benzphetamine hydrochloride tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:71335-0451(NDC:10702-040) Route of Administration ORAL DEA Schedule CIII Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZPHETAMINE HYDROCHLORIDE (UNII: 43DWT87QT7) (BENZPHETAMINE - UNII:0M3S43XK27) BENZPHETAMINE HYDROCHLORIDE 50 mg Inactive Ingredients Ingredient Name Strength CARNAUBA WAX (UNII: R12CBM0EIZ) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) FD&C RED NO. 40 (UNII: WZB9127XOA) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) POLYETHYLENE GLYCOL 3350 (UNII: G2M7P15E5P) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color orange (PEACH) Score 2 pieces Shape ROUND Size 10mm Flavor Imprint Code K;40 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71335-0451-1 30 in 1 BOTTLE; Type 0: Not a Combination Product 08/17/2018 2 NDC:71335-0451-2 7 in 1 BOTTLE; Type 0: Not a Combination Product 07/25/2018 3 NDC:71335-0451-3 14 in 1 BOTTLE; Type 0: Not a Combination Product 07/25/2018 4 NDC:71335-0451-4 21 in 1 BOTTLE; Type 0: Not a Combination Product 09/14/2018 5 NDC:71335-0451-5 42 in 1 BOTTLE; Type 0: Not a Combination Product 04/23/2018 6 NDC:71335-0451-6 84 in 1 BOTTLE; Type 0: Not a Combination Product 09/23/2022 7 NDC:71335-0451-7 60 in 1 BOTTLE; Type 0: Not a Combination Product 07/10/2018 8 NDC:71335-0451-8 90 in 1 BOTTLE; Type 0: Not a Combination Product 02/12/2018 9 NDC:71335-0451-9 120 in 1 BOTTLE; Type 0: Not a Combination Product 05/29/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA090968 07/21/2010 Labeler - Bryant Ranch Prepack (171714327) Registrant - Bryant Ranch Prepack (171714327) Establishment Name Address ID/FEI Business Operations Bryant Ranch Prepack 171714327 REPACK(71335-0451) , RELABEL(71335-0451)