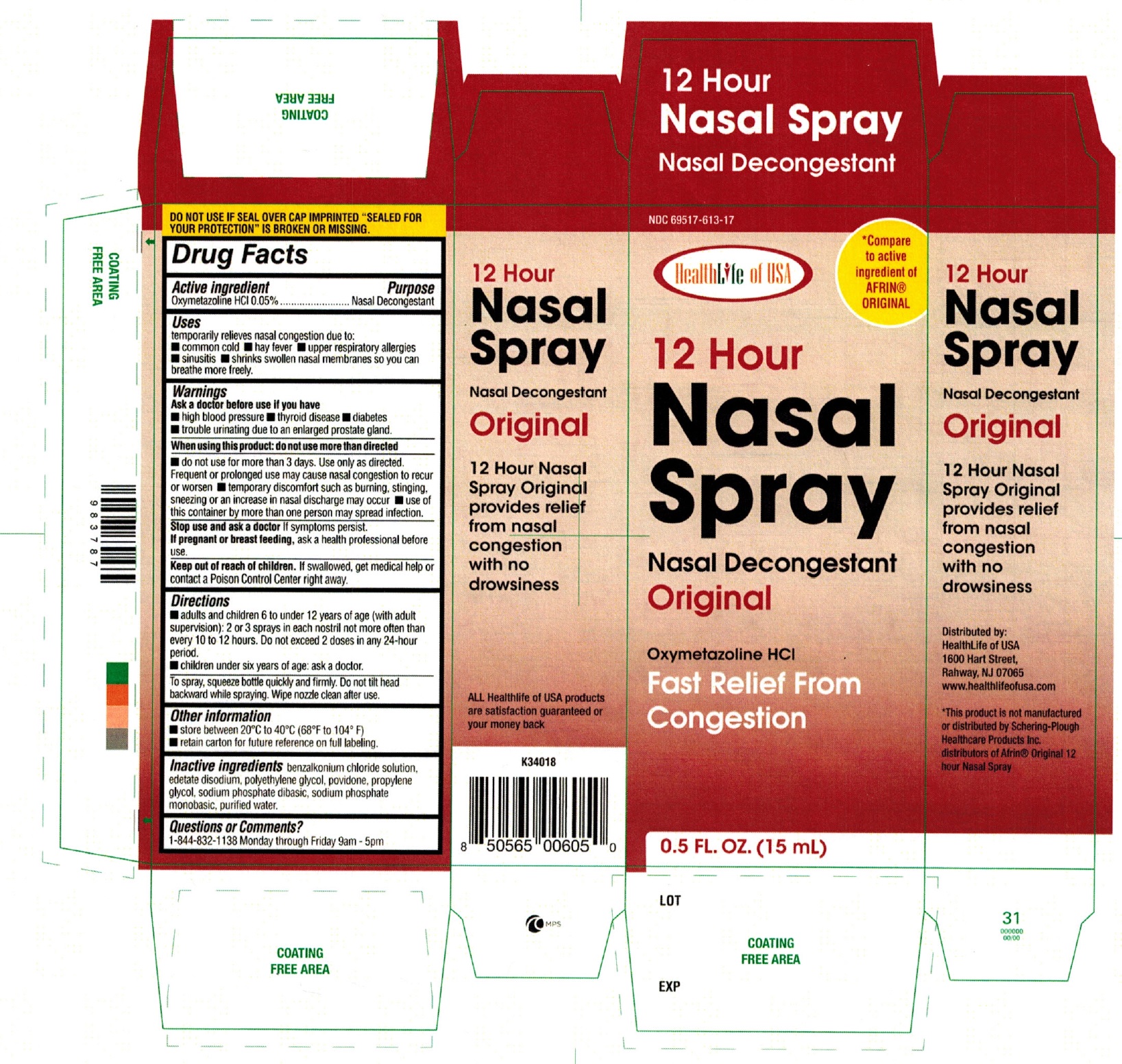
12 HOUR NASAL- oxymetazoline hcl spray
Healthlife of USA
----------
12 Hour Nasal Spray
Nasal Decongestant
Uses
Temporarily relieves nasal congestion due to:
- common cold
- hay fever
- upper respiratory allergies
- sinusitis
- shrinks swollen nasal membranes so you can breathe more freely
Warnings
Ask a doctor before use if you have
- high blood pressure
- thyroid disease
- diabetes
- trouble urinating due to an enlarged prostate gland
When using this product: do not use more than directed
- do not use for more than 3 days. use only as directed. Frequent or prolonged use may cause nasal congestion to recur or worsen
- temporary discomfort such as burning, stinging, sneezing or an increase in nasal discharge may occur
- use of this container by more than one person may spread infection.
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- adults and children 6 to under 12 years of age (with adult supervision): 2 or 3 sprays in each nostril not more often than every 10 to 12 hours. Do not exceed 2 doses in any 24-hour period.
- children under six years of age: ask a doctor.
- To spray, squeeze bottle quickly and firmly. Do not tilt head backward while spraying. Wipe nozzle clean after use.
Other Information
- store between 20°C to 40°C (68°F to 104°F)
- retain carton for future reference on full labeling.
| 12 HOUR NASAL
oxymetazoline hcl spray |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Healthlife of USA (079656178) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Kingston Pharmaceuticals | 080386521 | manufacture(69517-613) | |
Revised: 10/2023
Document Id: 0846bd38-5be2-d42b-e063-6294a90a87f2
Set id: 1c538da1-a005-4d05-9e59-d21c0882a676
Version: 2
Effective Time: 20231021
Healthlife of USA