ALOMIDE- lodoxamide tromethamine solution/ drops
Alcon Laboratories, Inc.
----------
ALOMIDE®
(lodoxamide tromethamine ophthalmic solution) 0.1%
DESCRIPTION:
ALOMIDE® (lodoxamide tromethamine ophthalmic solution) 0.1% is a sterile ophthalmic solution containing the mast cell stabilizer lodoxamide tromethamine for topical administration to the eyes. Lodoxamide tromethamine is a white, crystalline, water-soluble powder with a molecular weight of 553.91 g/mol. The chemical structure is presented below:
Structural Formula:
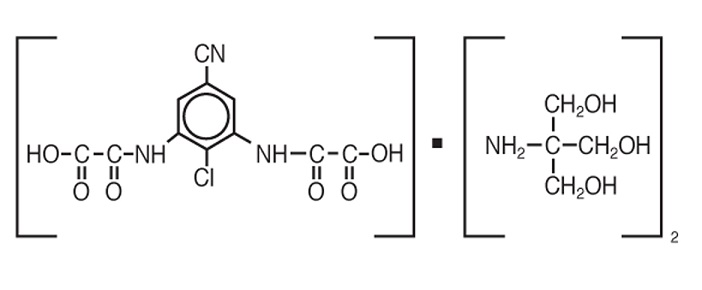
Chemical Name:
N,N'-(2-chloro-5-cyano-m-phenylene) dioxamic acid tromethamine salt
Molecular Formula: C19H28O12N5Cl
Each mL of ALOMIDE® (lodoxamide tromethamine ophthalmic solution) 0.1% contains: Active: 1.78 mg lodoxamide tromethamine equivalent to 1 mg lodoxamide.
Preservative: benzalkonium chloride 0.007%. Inactives: citric acid, edetate disodium, hydrochloric acid and/or sodium hydroxide (adjust pH), hypromellose 2910, mannitol, purified water, sodium citrate, and tyloxapol.
CLINICAL PHARMACOLOGY:
Lodoxamide tromethamine is a mast cell stabilizer that inhibits the in vivo Type I immediate hypersensitivity reaction. Lodoxamide therapy inhibits the increases in cutaneous vascular permeability that are associated with reagin or IgE and antigen-mediated reactions.
In vitro studies have demonstrated the ability of lodoxamide to stabilize rodent mast cells and prevent antigen-stimulated release of histamine. In addition, lodoxamide prevents the release of other mast cell inflammatory mediators (i.e., SRS-A, slow-reacting substances of anaphylaxis, also known as the peptido-leukotrienes) and inhibits eosinophil chemotaxis. Although lodoxamide's precise mechanism of action is unknown, the drug has been reported to prevent calcium influx into mast cells upon antigen stimulation.
Lodoxamide has no intrinsic vasoconstrictor, antihistaminic, cyclooxygenase inhibition, or other anti-inflammatory activity.
The disposition of 14C-lodoxamide was studied in six healthy adult volunteers receiving a 3 mg (50 μCi) oral dose of lodoxamide. Urinary excretion was the major route of elimination. The elimination half-life of 14C-lodoxamide was 8.5 hours in urine. In a study conducted in twelve healthy adult volunteers, topical administration of ALOMIDE® (lodoxamide tromethamine ophthalmic solution) 0.1%, one drop in each eye four times per day for ten days, did not result in any measurable lodoxamide plasma levels at a detection limit of 2.5 ng/mL.
INDICATIONS AND USAGE:
ALOMIDE® (lodoxamide tromethamine ophthalmic solution) 0.1% is indicated in the treatment of the ocular disorders referred to by the terms vernal keratoconjunctivitis, vernal conjunctivitis, and vernal keratitis.
WARNINGS:
FOR TOPICAL OPHTHALMIC USE ONLY. NOT FOR INJECTION. As with all ophthalmic preparations containing benzalkonium chloride, patients should be instructed not to wear soft contact lenses during treatment with ALOMIDE® (lodoxamide tromethamine ophthalmic solution) 0.1%. Do not touch the dropper tip to any surface, as this may contaminate the solution.
PRECAUTIONS:
General:
Patients may experience a transient burning or stinging upon instillation of ALOMIDE® (lodoxamide tromethamine ophthalmic solution) 0.1%. Should these symptoms persist, the patient should be advised to contact the prescribing physician.
Carcinogenesis, Mutagenesis, Impairment of Fertility:
A long-term study with lodoxamide tromethamine in rats (two-year oral administration) showed no neoplastic or tumorigenic effects at doses 100 mg/kg/day (more than 5000 times the proposed human clinical dose). No evidence of mutagenicity or genetic damage was seen in the Ames Salmonella Assay, Chromosomal Aberration in CHO Cells Assay, or Mouse Forward Lymphoma Assay. In the BALB/c-3T3 Cells Transformation Assay, some increase in the number of transformed foci was seen at high concentrations (greater than 4,000 mcg/mL). No evidence of impairment of reproductive function was shown in laboratory animal studies.
Pregnancy:
Reproduction studies with lodoxamide tromethamine administered orally to rats and rabbits in doses of 100 mg/kg/day (more than 5000 times the proposed human clinical dose) produced no evidence of developmental toxicity. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, ALOMIDE® (lodoxamide tromethamine ophthalmic solution) 0.1% should be used during pregnancy only if clearly needed.
Nursing Mothers:
It is not known whether lodoxamide tromethamine is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when ALOMIDE® (lodoxamide tromethamine ophthalmic solution) 0.1% is administered to nursing women.
ADVERSE REACTIONS:
During clinical studies of ALOMIDE® (lodoxamide tromethamine ophthalmic solution) 0.1%, the most frequently reported ocular adverse experiences were transient burning, stinging, or discomfort upon instillation, which occurred in approximately 15% of the subjects. Other ocular events occurring in 1% to 5% of the subjects included ocular itching/pruritus, blurred vision, dry eye, tearing/discharge, hyperemia, crystalline deposits, and foreign body sensation. Events that occurred in less than 1% of the subjects included corneal erosion/ulcer, scales on lid/lash, eye pain, ocular edema/swelling, ocular warming sensation, ocular fatigue, chemosis, corneal abrasion, anterior chamber cells, keratopathy/keratitis, blepharitis, allergy, sticky sensation, and epitheliopathy.
Nonocular events reported were headache (1.5%) and (at less than 1%) heat sensation, dizziness, somnolence, nausea, stomach discomfort, sneezing, dry nose, and rash.
OVERDOSAGE:
There have been no reports of ALOMIDE® (lodoxamide tromethamine ophthalmic solution) 0.1% overdose following topical ocular application. Accidental overdose of an oral preparation of 120 mg to 180 mg of lodoxamide resulted in a temporary sensation of warmth, profuse sweating, diarrhea, light-headedness, and a feeling of stomach distension; no permanent adverse effects were observed. Side effects reported following systemic oral administration of 0.1 mg to 10 mg of lodoxamide include a feeling of warmth or flushing, headache, dizziness, fatigue, sweating, nausea, loose stools, and urinary frequency/urgency. The physician may consider emesis in the event of accidental ingestion.
DOSAGE AND ADMINISTRATION:
The dose for adults and children greater than 2 years of age is one to two drops in each affected eye four times daily for up to 3 months.
HOW SUPPLIED:
ALOMIDE® (lodoxamide tromethamine ophthalmic solution) 0.1% is supplied in a plastic ophthalmic dispenser as follows:
10 mL NDC 0065-0345-10
Storage:
Store at 20°C to 25°C (68°F to 77°F), excursions permitted between 15°C and 30°C (59°F and 86°F) [see USP Controlled Room Temperature].
| ALOMIDE
lodoxamide tromethamine solution/ drops |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - Alcon Laboratories, Inc. (008018525) |
