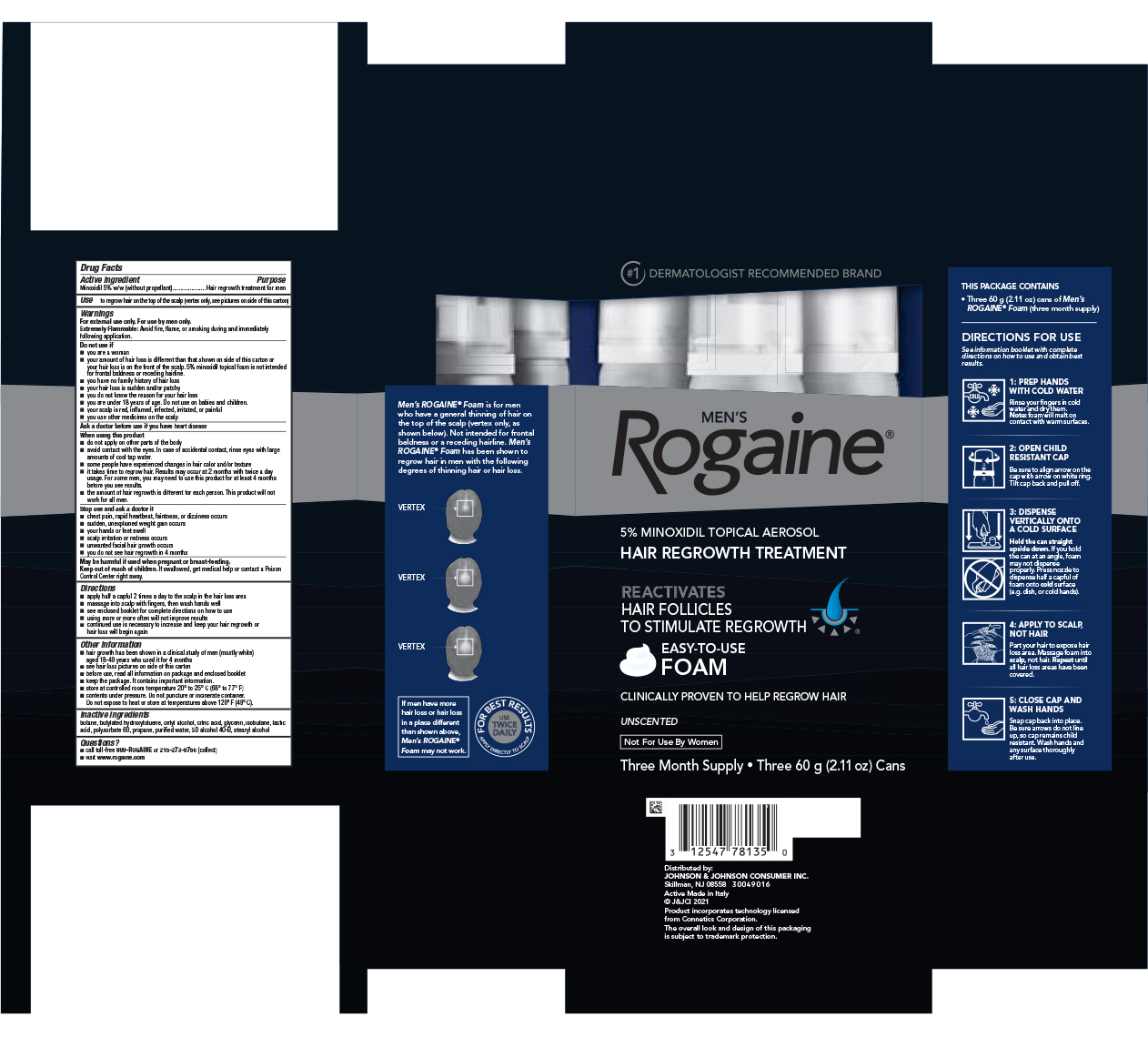
Label: MENS ROGAINE MINOXIDIL - UNSCENTED FORMULA- minoxidil aerosol, foam
- NDC Code(s): 69968-0031-1, 69968-0031-3, 69968-0031-4
- Packager: Johnson & Johnson Consumer Inc.
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated January 11, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Use
-
Warnings
For external use only. For use by men only.
Do not Use if
- you are a woman
- your amount of hair loss is different than that shown on side of this carton or your hair loss is on the front of the scalp. 5% minoxidil topical foam is not intended for frontal baldness or receding hairline.
- you have no family history of hair loss
- your hair loss is sudden and/or patchy
- you do not know the reason for your hair loss
- you are under 18 years of age. Do not use on babies and children.
- your scalp is red, inflamed, infected, irritated, or painful
- you use other medicines on the scalp
When using this product
- do not apply on other parts of the body
- avoid contact with the eyes. In case of accidental contact, rinse eyes with large amounts of cool tap water.
- some people have experienced changes in hair color and/or texture
- it takes time to regrow hair. Results may occur at 2 months with twice a day usage. For some men, you may need to use this product for at least 4 months before you see results.
- the amount of hair regrowth is different for each person. This product will not work for all men.
-
Directions
- apply half a capful 2 times a day to the scalp in the hair loss area
- massage into scalp with fingers, then wash hands well
- see enclosed booklet for complete directions on how to use
- using more or more often will not improve results
- continued use is necessary to increase and keep your hair regrowth or hair loss will begin again
-
Other information
- hair growth has been shown in a clinical study of men (mostly white) aged 18-49 years who used it for 4 months
- see hair loss pictures on side of this carton
- before use, read all information on package and enclosed booklet
- keep the package. It contains important information
- store at controlled room temperature 20° to 25° C (68° to 77°F)
- contents under pressure. Do not puncture or incinerate container. Do not expose to heat or store at temperatures above 120°F (49°C).
- Inactive ingredients
- Questions?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - Three 60 g Can Package
-
INGREDIENTS AND APPEARANCE
MENS ROGAINE MINOXIDIL - UNSCENTED FORMULA
minoxidil aerosol, foamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69968-0031 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MINOXIDIL (UNII: 5965120SH1) (MINOXIDIL - UNII:5965120SH1) MINOXIDIL 50 mg in 1 g Inactive Ingredients Ingredient Name Strength BUTANE (UNII: 6LV4FOR43R) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) CETYL ALCOHOL (UNII: 936JST6JCN) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) GLYCERIN (UNII: PDC6A3C0OX) ISOBUTANE (UNII: BXR49TP611) LACTIC ACID, UNSPECIFIED FORM (UNII: 33X04XA5AT) POLYSORBATE 60 (UNII: CAL22UVI4M) PROPANE (UNII: T75W9911L6) WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) STEARYL ALCOHOL (UNII: 2KR89I4H1Y) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0031-1 1 in 1 PACKAGE 10/01/2010 1 60 g in 1 CAN; Type 0: Not a Combination Product 2 NDC:69968-0031-3 3 in 1 PACKAGE 10/01/2010 2 60 g in 1 CAN; Type 0: Not a Combination Product 3 NDC:69968-0031-4 4 in 1 PACKAGE 10/01/2010 3 60 g in 1 CAN; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021812 10/01/2010 Labeler - Johnson & Johnson Consumer Inc. (118772437)