MONS- glycerin, allantoin gel
Pharmacal-International. Co., Ltd
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
MONS GEL
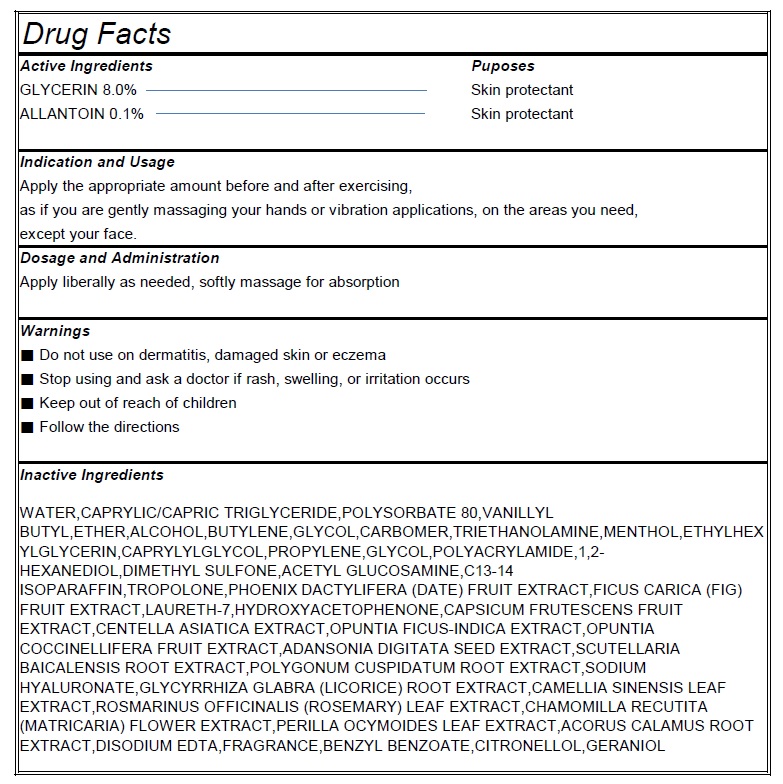
Active Ingredient Purpose
GLYCERIN 8.0%------------------------- Skin protectant
ALLANTOIN 0.1%----------------------- Skin protectant
Indication and Usage
Apply the appropriate amount before and after exercising,
as if you are gently massaging your hands or vibration applications, on the areas you need,
except your face.
Warnings
■ Do not use on dermatitis, damaged skin or eczema
■ Stop using and ask a doctor if rash, swelling, or irritation occurs
■ Keep out of reach of children
■ Follow the directions
Inactive ingrdients
WATER,CAPRYLIC/CAPRIC TRIGLYCERIDE,POLYSORBATE 80,VANILLYL BUTYL,ETHER,ALCOHOL,BUTYLENE,GLYCOL,CARBOMER,TRIETHANOLAMINE,MENTHOL,ETHYLHEXYLGLYCERIN,CAPRYLYLGLYCOL,PROPYLENE,GLYCOL,POLYACRYLAMIDE,1,2-HEXANEDIOL,DIMETHYL SULFONE,ACETYL GLUCOSAMINE,C13-14 ISOPARAFFIN,TROPOLONE,PHOENIX DACTYLIFERA (DATE) FRUIT EXTRACT,FICUS CARICA (FIG) FRUIT EXTRACT,LAURETH-7,HYDROXYACETOPHENONE,CAPSICUM FRUTESCENS FRUIT EXTRACT,CENTELLA ASIATICA EXTRACT,OPUNTIA FICUS-INDICA EXTRACT,OPUNTIA COCCINELLIFERA FRUIT EXTRACT,ADANSONIA DIGITATA SEED EXTRACT,SCUTELLARIA BAICALENSIS ROOT EXTRACT,POLYGONUM CUSPIDATUM ROOT EXTRACT,SODIUM HYALURONATE,GLYCYRRHIZA GLABRA (LICORICE) ROOT EXTRACT,CAMELLIA SINENSIS LEAF EXTRACT,ROSMARINUS OFFICINALIS (ROSEMARY) LEAF EXTRACT,CHAMOMILLA RECUTITA (MATRICARIA) FLOWER EXTRACT,PERILLA OCYMOIDES LEAF EXTRACT,ACORUS CALAMUS ROOT EXTRACT,DISODIUM EDTA,FRAGRANCE,BENZYL BENZOATE,CITRONELLOL,GERANIOL.
| MONS
glycerin, allantoin gel |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Pharmacal-International. Co., Ltd (557805060) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| EcoWorldpharm Co., Ltd. | 688735061 | manufacture(24765-125) | |