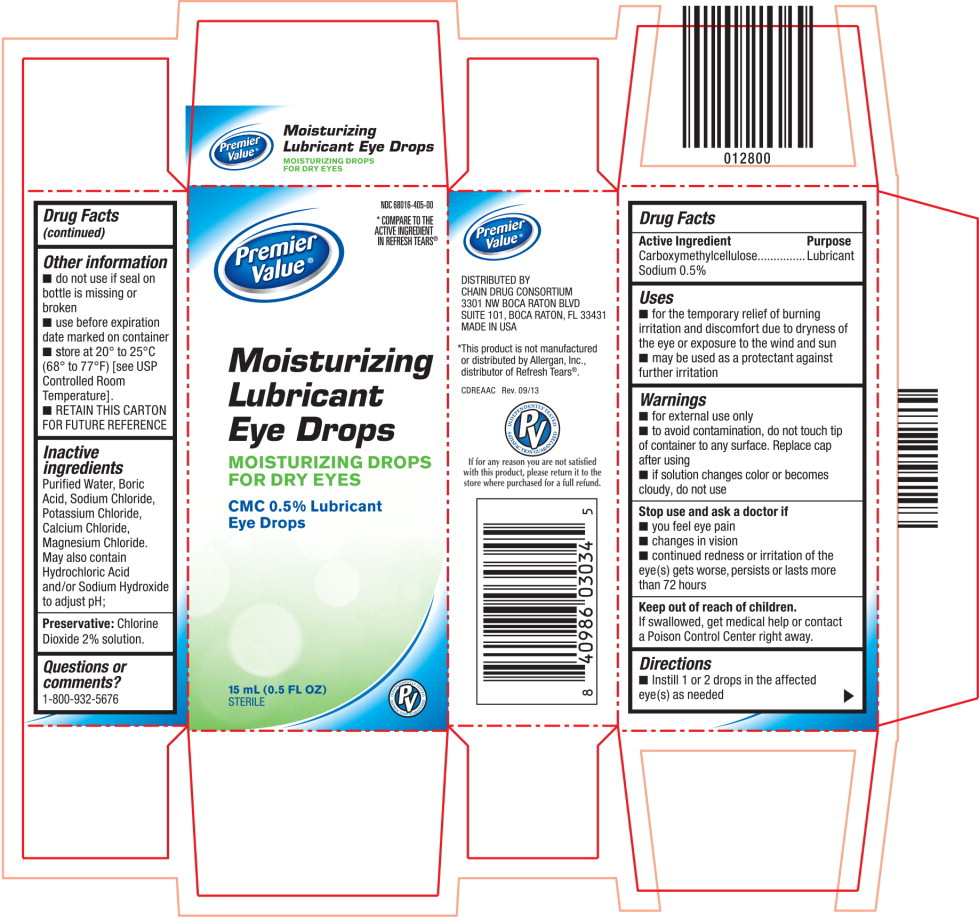
MOISTURIZING LUBRICANT EYE DROPS- carboxymethylcellulose sodium solution/ drops
Chain Drug Consortium
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Uses
- for the temporary relief of burning irritation and discomfort due to dryness of the eye or exposure to the wind and sun
- may be used as a protectant against further irritation
Warnings
- for external use only
- to avoid contamination, do not touch tip of container to any surface. Replace cap after using
- if solution changes color or becomes cloudy, do not use
Other information
- do not use if seal on bottle is missing or broken
- use before expiration date marked on container
- store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
- RETAIN THIS CARTON FOR FUTURE REFERENCE
Inactive ingredients
Purified Water, Boric Acid, Sodium Chloride, Potassium Chloride, Calcium Chloride, Magnesium Chloride.
May also contain Hydrochloric Acid and/or Sodium Hydroxide to adjust pH;
| MOISTURIZING LUBRICANT EYE DROPS
carboxymethylcellulose sodium solution/ drops |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - Chain Drug Consortium (101668460) |
Revised: 10/2020
Document Id: 891e70b3-5819-4bb9-bf21-1562376f3ec4
Set id: 1af7e6c4-b25f-4edc-a625-a3e2ee0c9f51
Version: 3
Effective Time: 20201021
Chain Drug Consortium