A4096 EPIDURAL
- regional anesthesia kit
Smiths Medical ASD, Inc.
----------
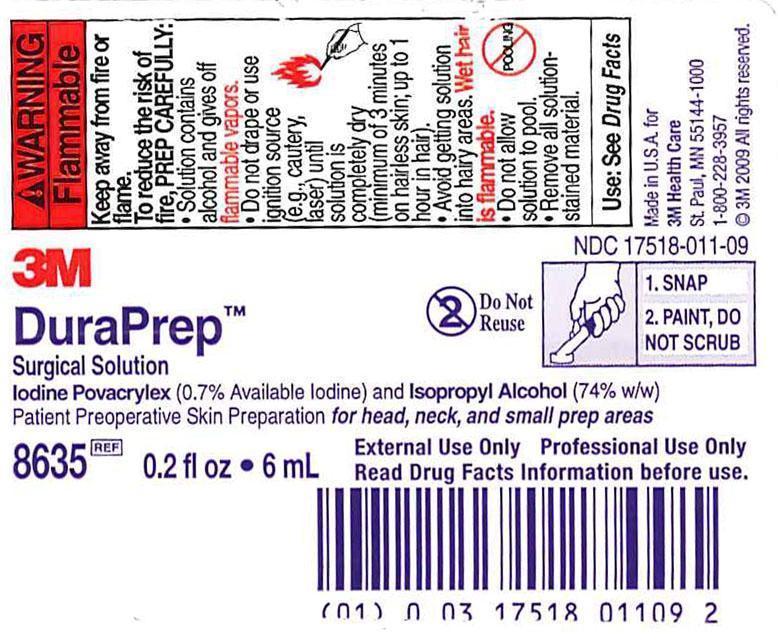
Antiseptic
patient preoperative skin preparation:
for preparation of the skin prior to surgeryhelps reduce bacteria that potentially can cause skin infection
For external use only. Flammable, keep away from fire or flame.
To reduce the risk of fire, PREP CAREFULLY:
solution contains alcohol and gives off flammable vaporsdo not drape or use ignition source (e.g., cautery, laser) until solution is completely dry (minimum of 3 minutes on hairless skin; up to 1 hour in hair).avoid getting solution into hairy areas. Wet hair is flammable. Hair may take up to 1 hour to dry.do not allow solution to poolremove solution-stained material from prep area

Do not use
on patients with known allergies to iodine or any other ingredients in this producton open wounds, on mucous membranes, or as a general skin cleanserin infants less than 2 months old due to the risk of excessive skin irritation and transient hypothyroidism
When using this product
keep out of eyes, ears, and mouth. May cause serious injury if permitted to enter and remain. If contact occurs, flush with cold water right away and contact a doctor.to avoid skin injury, care should be taken when removing drapes, tapes, etc…applied over filmuse with caution in women who are breast-feeding due to the potential for transient hypothyroidism in the nursing newborn
Stop use and ask a doctor if irritation, sensitization or allergic reaction occurs. These may be signs of a serious condition. On rare occasions, use of this product has been associated with skin blistering.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
at the end of the prep, discard any portion of the solution which is not required to cover the prep area. It is not necessary to use the entire amount available.
Getting Patient Ready for Solution:
use in well-ventilated areado not microwave or heat the solution applicatorapply to clean, completely dry, residue-free, intact skinwhen hair removal is necessary, use a surgical clipper on the morning of the surgery. If a wet shave is used, thoroughly remove all soap residues.
Activating the Applicator:
grasp product by wrapping hand and fingers around the labeled portion of the applicator. Place thumb on the lever.with sponge face parallel to the floor, snap lever. Allow all fluid to flow into sponge.
When Applying Solution:
DO NOT SCRUB. Paint a single, uniform application and do not reprep area.do not allow solution to pool. Use sponge applicator to absorb excess solution and continue to apply a uniform coating. If solution accidentally gets outside of prep area, remove excess with gauze.tuck prep towels as needed under both sides of the neck to absorb excess solution. Remove towels before draping.avoid getting solution into hairy areas. Wet hair is flammable. Hair may take up to 1 hour to dry.when prepping skin folds, toes, or fingers, use a sterile-gloved hand to hold skin apart until completely dry. Otherwise, skin may adhere to itself.

After Applying Solution:
to reduce the risk of fire, wait until solution is completely dry (minimum of 3 minutes on hairless skin; up to 1 hour in hair). Solution will turn from a shiny to a dull appearance on skin alerting the user that the solution is completely dry and no longer flammable.
While Waiting for Solution to Completely Dry:
do not drape or use ignition source (e.g., cautery, laser)check for pooled solution. Use sterile gauze to soak up pooled solution. Do not blot because it may remove solution from skin.remove solution-stained materials. Replace if necessary.

After Solution is Completely Dry:
to reduce the risk of fire, begin draping and/or using cautery only after solution is completely dry and all solution-stained materials are removedif incise drapes are used, apply directly to dry prep. On completion of surgical procedure, removal of incise drape will remove film.apply dressing following standard practices
| A4096 EPIDURAL
regional anesthesia kit kit |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Smiths Medical ASD, Inc. (137835299) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Smiths Medical ASD, Inc. | 137835299 | manufacture | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| 3M Company | 078671244 | manufacture | |