ALPROLIX- coagulation factor ix (recombinant), fc fusion protein
Biogen Inc.
----------
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ALPROLIX® safely and effectively. see full prescribing information for ALPROLIX.
ALPROLIX® [Coagulation Factor IX (Recombinant), Fc Fusion Protein], Lyophilized Powder for Solution For Intravenous Injection. Initial U.S. Approval: 2014 INDICATIONS AND USAGEALPROLIX, Coagulation Factor IX (Recombinant), Fc Fusion Protein, is a recombinant DNA derived, coagulation Factor IX concentrate indicated in adults and children with hemophilia B for:
Limitations of Use ALPROLIX is not indicated for induction of immune tolerance in patients with hemophilia B. (1) DOSAGE AND ADMINISTRATIONFor intravenous use after reconstitution only. On demand treatment and control of bleeding episodes and perioperative management of bleeding:
Routine prophylaxis: 50 IU/kg once weekly or 100 IU/kg once every 10 days. Adjust dosing regimen based on individual response. (2.1) DOSAGE FORMS AND STRENGTHSALPROLIX is available as a lyophilized powder in single use vials containing nominally 250, 500, 1000, 2000, 3000, or 4000 international units (IU). (3) CONTRAINDICATIONSDo not use in individuals who have a known history of hypersensitivity reactions, including anaphylaxis, to the product or its excipients. (4) WARNINGS AND PRECAUTIONS
ADVERSE REACTIONSCommon adverse reactions (incidence ≥1%) from clinical trials were headache and oral paresthesia. (6) To report SUSPECTED ADVERSE REACTIONS, contact Biogen Inc. at 1-855-692-5776 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling. Revised: 4/2017 |
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
ALPROLIX, Coagulation Factor IX (Recombinant), Fc Fusion Protein, is a recombinant DNA derived, coagulation Factor IX concentrate indicated in adults and children with hemophilia B (congenital Factor IX deficiency) for:
- On demand treatment and control of bleeding episodes,
- Perioperative management of bleeding,
- Routine prophylaxis to reduce the frequency of bleeding episodes.
Limitation of Use
ALPROLIX is not indicated for induction of immune tolerance in patients with hemophilia B [see Warnings and Precautions (5.3)].
2 DOSAGE AND ADMINISTRATION
For intravenous use after reconstitution only
2.1 Dosing Guidelines
- Initiate treatment with ALPROLIX under the supervision of a qualified healthcare professional experienced in the treatment of hemophilia B.
- Dose and duration of treatment depend on the severity of the Factor IX deficiency, the location and extent of bleeding, and the patient's clinical condition.
- Patients may vary in their pharmacokinetic (e.g., half-life, in vivo recovery) and clinical responses. Base the dose and frequency of ALPROLIX on the individual clinical response. Each vial label for ALPROLIX states the Factor IX potency in international units (IU). ALPROLIX potency is assigned using an in vitro, activated partial thromboplastin time (aPTT)-based, one-stage clotting assay calibrated against the World Health Organization (WHO) international standard for Factor IX concentrates.
- Factor IX activity measurements in the clinical laboratory may be affected by the type of aPTT reagent or laboratory standard used.[see Warnings and Precautions (5.4)]
One IU of ALPROLIX per kg body weight increases the circulating level of Factor IX by 1% [IU/dL]. Estimate the required dose or the expected in vivo peak increase in Factor IX level expressed as IU/dL (or % of normal) using the following formulas:
| IU/dL (or % of normal) = |
| [Total Dose (IU)/Body Weight (kg)] x Recovery (IU/dL per IU/kg) |
| OR |
| Dose (IU) = |
| Body Weight (kg) x Desired Factor IX Rise (IU/dL or, |
| % of normal) x Reciprocal of Recovery (IU/kg per IU/dL) |
- Dose adjustment may be necessary in pediatric patients under 12 years of age [see Use in Specific Populations (8.4)]. For patients 12 years of age or older, dose adjustment is not usually required.
On Demand Treatment and Control of Bleeding Episodes
ALPROLIX dosing for the on demand treatment and control of bleeding episodes is provided in Table 1.
| Type of Bleeding | Circulating Factor IX Level
Required (IU/dL or % of normal) | Dosing Interval (hours) |
|---|---|---|
| Minor and Moderate
For example: Uncomplicated hemarthroses, superficial muscle (except iliopsoas) without neurovascular compromise, superficial soft tissue, mucous membranes | 30-60 | Repeat every 48 hours if there is further evidence of bleeding |
| Major
For example: Iliopsoas and deep muscle with neurovascular injury, or substantial blood loss; Pharyngeal, retropharyngeal, retroperitoneal, CNS | 80-100 | Consider a repeat dose after 6-10 hours and then every 24 hours for the first 3 days. Due to the long half-life of ALPROLIX, the dose may be reduced and frequency of dosing may be extended after day 3 to every 48 hours or longer until bleeding stops and healing is achieved. |
Perioperative Management
ALPROLIX dosing for perioperative management is provided in Table 2.
| Type of Surgery | Circulating Factor IX Level Required (IU/dL or % of normal) | Dosing Interval (hours) |
|---|---|---|
| Minor
(including uncomplicated dental extraction) | 50 to 80 | A single infusion may be sufficient. Repeat as needed after 24-48 hours until bleeding stops and healing is achieved. |
| Major | 60 to 100 (initial level) | Consider a repeat dose after 6-10 hours and then every 24 hours for the first 3 days. Due to the long half-life of ALPROLIX, the dose may be reduced and frequency of dosing in the post-surgical setting may be extended after day 3 to every 48 hours or longer until bleeding stops and healing is achieved. |
2.2 Reconstitution
- Use aseptic technique (clean and germ-free) and a flat work surface during the reconstitution procedure.
- Allow the vial of ALPROLIX and the pre-filled diluent syringe to reach room temperature before use.
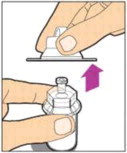
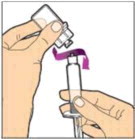
- Remove the plastic cap from the vial and wipe the rubber stopper of the vial with an alcohol wipe. Allow the rubber stopper to dry.
-
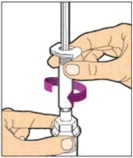
Completely remove the backing from the vial adapter package by peeling back the lid. Do not remove the vial adapter from the package or touch the inside of the package of the adapter.
-
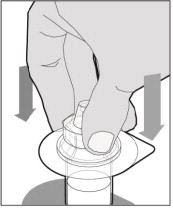
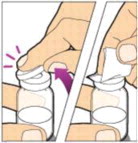
Place the vial on a flat surface and use one hand to hold the vial steady. Use the other hand to place the vial adapter over the vial. Place the adapter spike directly above the center of the rubber stopper and push the adapter straight down until the spike punctures the center of the vial stopper and is fully inserted.
-
Lift the package cover away from the vial adapter and discard the cover.
-
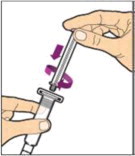
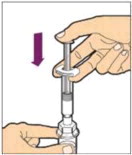
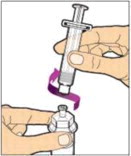
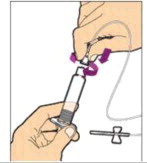
Hold the plunger rod at the circular disk. Place the tip of the plunger rod into the end of the syringe. Turn clockwise until it is securely attached. Only use the diluent syringe provided in the ALPROLIX package.
- With one hand, hold the diluent syringe by the ridged part directly under the cap, with the cap pointing up. Do not use if the cap has been removed or is not securely attached.
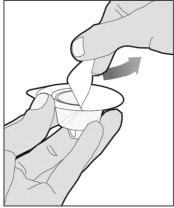
- With your other hand, grasp the cap and bend it at a 90° angle until it snaps off. After the cap snaps off, you will see the glass tip of the syringe. Do not touch the glass tip of the syringe or the inside of the cap.
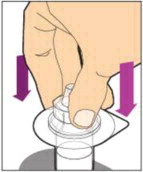
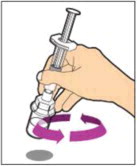
- With the vial sitting on a flat surface, insert the tip of the syringe into the adapter opening. Turn the syringe clockwise until it is securely attached to the adapter.
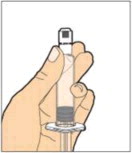
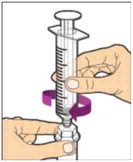
- Slowly depress the plunger rod to inject all of the diluent into the vial. The plunger rod may rise slightly after this process. This is normal.
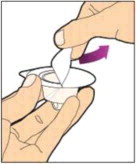
- With the syringe still connected to the adapter, gently swirl the vial until the product is completely dissolved. The final solution should be clear to slightly opalescent and colorless. Do not shake. Do not use the reconstituted ALPROLIX if it contains visible particles or is cloudy.
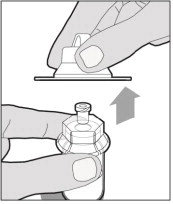
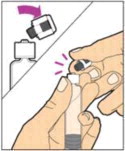
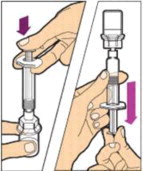
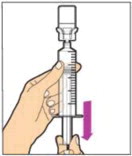
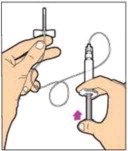
- Make sure the plunger rod is completely depressed. Turn the vial upside-down. Slowly pull on the plunger rod to draw the solution into the syringe. Be careful not to pull the plunger rod completely out of the syringe.
- Gently unscrew the syringe from the vial adapter and dispose of the vial with the adapter still attached. Do not touch the syringe tip or the inside of the cap.
- Use the reconstituted ALPROLIX as soon as possible, but no later than 3 hours after reconstitution. Protect from direct sunlight. Do not refrigerate after reconstitution.
To combine two or more vials of ALPROLIX, after step 12 above, follow these pooling steps:
- Remove the diluent syringe from the vial adapter by turning it counterclockwise until it is completely detached. Do not detach the diluent syringe or the large luer lock syringe until ready to attach the large luer lock syringe to the next vial (with vial adapter attached).
- Leave the vial adapter attached to the vial, as it is needed for attaching a large luer lock syringe.
- Attach a separate, large luer lock syringe by turning clockwise until it is securely in place.
- Slowly pull on the plunger rod to draw the solution into the syringe.
- Repeat this pooling procedure with each vial necessary to obtain the required dose. Once you have pooled the required dose, proceed to administration using the large luer lock syringe.
2.3 Administration
For intravenous injection only
- Inspect the reconstituted ALPROLIX solution visually for particulate matter and discoloration prior to administration. Do not use if particulate matter or discoloration is observed.
- Do not administer reconstituted ALPROLIX in the same tubing or container with other medications.
Administration Steps:
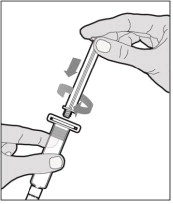
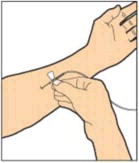
- Attach the syringe to the connector end of the infusion set tubing by turning clockwise until it is securely in place.
- Depress the plunger until all air is removed from the syringe and ALPROLIX has reached the end of the infusion set tubing. Do not push ALPROLIX through the needle.
- Remove the protective needle cover from the infusion set tubing.
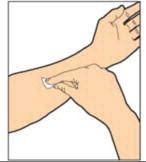
- Perform intravenous bolus infusion. The rate of administration should be determined by the patient's comfort level, and no faster than 10 ml per minute.
After infusing ALPROLIX, remove and properly discard the infusion set.
3 DOSAGE FORMS AND STRENGTHS
ALPROLIX is available as a lyophilized powder in single use vials containing nominally 250, 500, 1000, 2000, 3000, or 4000 international units (IU) per vial. The actual Factor IX potency is stated on each ALPROLIX vial.
4 CONTRAINDICATIONS
ALPROLIX is contraindicated in individuals who have a known history of hypersensitivity reactions, including anaphylaxis, to the product or its excipients (sucrose, mannitol, sodium chloride, L-histidine and polysorbate 20) .
.
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
Hypersensitivity reactions have been reported with ALPROLIX. Allergic-type hypersensitivity reactions, including anaphylaxis, have been reported with Factor IX replacement products.
The presence of inhibitors has been associated with allergic reactions with Factor IX replacement therapies, including with ALPROLIX. Evaluate patients experiencing allergic reactions for the presence of an inhibitor. Early signs of allergic reactions, which can progress to anaphylaxis, may include angioedema, chest tightness, hypotension, rash, nausea, vomiting, paresthesia, restlessness, wheezing and dyspnea. Discontinue use of ALPROLIX if hypersensitivity symptoms occur, and initiate appropriate treatment.
5.2 Neutralizing Antibodies
Formation of neutralizing antibodies (inhibitors) to Factor IX has been reported following administration of ALPROLIX, including in previously untreated patients. Monitor all patients regularly for the development of inhibitors by appropriate clinical observations and laboratory tests [see Warnings and Precautions (5.4)].
Evaluate patients experiencing allergic reactions for the presence of an inhibitor. Closely observe patients for signs and symptoms of acute hypersensitivity reactions, particularly during the early phases of exposure to the product.
Individuals with Factor IX inhibitors may be at an increased risk of anaphylaxis upon subsequent challenge with ALPROLIX.
5.3 Thromboembolic Complications
The use of Factor IX products has been associated with the development of thromboembolic complications, especially in individuals receiving continuous infusion through a central venous catheter. ALPROLIX should be administered as bolus infusion over several minutes [see Dosage and Administration (2.3)]. The safety of ALPROLIX administration by continuous infusion has not been studied.
5.4 Monitoring Laboratory Tests
- To confirm adequate Factor IX levels have been achieved and maintained, monitor plasma Factor IX activity by performing the one-stage clotting assay [see Dosage and Administration (2.1)]. Factor IX results can be affected by the type of aPTT reagent used. Measurement with a one-stage clotting assay using a kaolin-based aPTT reagent will likely result in an underestimation of activity level.
- Monitor for the development of Factor IX inhibitors if the expected Factor IX activity levels in plasma are not attained, or if bleeding is not controlled with the recommended dose of ALPROLIX. Perform a Bethesda assay to determine if Factor IX inhibitors are present.
6 ADVERSE REACTIONS
Common adverse reactions (incidence ≥1%) reported in clinical trials were headache and oral paresthesia.
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in clinical trials of another drug and may not reflect the rates observed in clinical practice.
In the multi-center, prospective, open-label clinical trial with ALPROLIX, 123 previously treated patients (PTPs, exposed to a Factor IX containing product for ≥100 exposure days) were evaluated, with 115 subjects treated for at least 26 weeks and 56 subjects treated for at least 52 weeks.
Adverse reactions (ARs) were reported in 10 of 119 (8.4%) subjects treated with routine prophylaxis or episodic (on-demand) therapy. They are summarized in Table 3.
No subject was withdrawn from the trial due to an adverse reaction. In the trial, no inhibitors were detected and no events of anaphylaxis were reported.
|
*119 previously treated patients (PTPs) on routine prophylaxis or episodic (on-demand) therapy |
||
| System Organ Class | Adverse Reactions (AR) | Number of Subjects (%)
N=119* |
| Nervous system disorders | Headache Dizziness Dysgeusia | 2 (1.7) 1 (0.8) 1 (0.8) |
| Gastrointestinal disorders | Paresthesia oral Breath odor | 2 (1.7) 1 (0.8) |
| General disorders and administration site conditions | Fatigue Infusion site pain | 1 (0.8) 1 (0.8) |
| Cardiac disorders | Palpitations | 1 (0.8) |
| Renal and urinary disorders | Obstructive uropathy | 1 (0.8) |
| Vascular disorders | Hypotension | 1 (0.8) |
Obstructive uropathy was reported in one subject with hematuria who developed an obstructing clot in the urinary collecting system. The event resolved with hydration and the subject continued prophylactic treatment with ALPROLIX. A causal relationship of clot formation to ALPROLIX was not established.
Immunogenicity
Previously-treated patients participating in clinical trials were monitored for neutralizing antibodies to Factor IX. No subjects developed neutralizing antibodies to Factor IX.
The detection of antibodies that are reactive to Factor IX is highly dependent on many factors, including the sensitivity and specificity of the assay, sample handling, timing of sample collection, concomitant medications and underlying disease.
6.2 Postmarketing Experience
The following adverse reaction has been identified during the post-approval use of ALPROLIX. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Blood and lymphatic system disorders: Factor IX inhibitor development
Immune system disorders: hypersensitivity
8 USE IN SPECIFIC POPULATIONS
8.2 Labor and Delivery
There is no information available on the effect of Factor IX replacement therapy on labor and delivery. Use only if the potential benefit justifies the potential risk.
8.3 Nursing Mothers
It is not known if ALPROLIX is excreted into human milk. Because many drugs are excreted in human milk, caution should be exercised if ALPROLIX is administered to nursing women.
8.4 Pediatric Use
Safety, efficacy, and pharmacokinetics of ALPROLIX have been evaluated in previously treated pediatric patients 12 years of age and older. No dose adjustment is required for adolescents.
Children under 12 years of age may have higher Factor IX body weight-adjusted clearance, shorter half-life, and lower recovery. Higher dose per kilogram body weight or more frequent dosing may be needed in these patients [see Clinical Pharmacology (12.3)].
The use of ALPROLIX in children younger than 12 years of age is supported by the clinical trial of ALPROLIX in subjects 12 years of age and older and interim pharmacokinetic and safety data from a trial of pediatric subjects including 8 subjects 2 to 5 years of age and 15 subjects 6 to 11 years of age who were exposed for a median of 21.3 weeks (1.1 to 45.7 weeks). The safety profile in subjects under 12 years of age is acceptable. Efficacy can be extrapolated from pharmacokinetic data to subjects < 2 years of age. [see Clinical Pharmacology (12.3)]
11 DESCRIPTION
Coagulation Factor IX (Recombinant), Fc Fusion Protein [rFIXFc], the active ingredient in ALPROLIX, is a recombinant coagulation Factor IX protein consisting of the human coagulation Factor IX sequence covalently linked to the Fc domain of human immunoglobulin G1 (IgG1). The Factor IX portion of rFIXFc has a primary amino acid sequence that is identical to the Thr148 allelic form of plasma derived Factor IX and has structural and functional properties similar to endogenous Factor IX. The Fc domain of rFIXFc contains the hinge, CH2, and CH3 regions of IgG1. rFIXFc contains 867 amino acids with a molecular weight of approximately 98 kilodaltons.
ALPROLIX is not derived from human blood and contains no preservatives. The recombinant Factor IX Fc fusion protein is expressed in a human embryonic kidney (HEK) cell line, which produces rFIXFc into a defined cell culture medium that does not contain proteins derived from animal or human sources. The purification process for rFIXFc does not include use of a monoclonal antibody reagent. To enhance viral safety, the production process also incorporates two dedicated viral clearance steps – a detergent treatment step for inactivation and a 15 nm filtration step for removal of viruses. The content of activated Factor IX Fc fusion protein (FIXaFc) is limited to ≤0.035 mole percent FIXaFc/FIXFc.
ALPROLIX is a sterile, non-pyrogenic, preservative-free, white to off-white, lyophilized powder to cake for reconstitution with the provided diluent, for intravenous injection. After reconstitution, the solution has a clear to slightly opalescent appearance and contains the excipients: sucrose, mannitol, sodium chloride, L-histidine and polysorbate 20. ALPROLIX is available in single-use vials containing the labeled amount of Factor IX activity, expressed in international units. Each vial contains nominally 250 IU, 500 IU, 1000 IU, 2000 IU 3000, or 4000 IU.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
ALPROLIX is a recombinant, fusion protein that temporarily replaces the missing coagulation Factor IX needed for effective hemostasis. ALPROLIX contains the Fc region of human IgG1, which binds to the neonatal Fc receptor (FcRn). FcRn is part of a naturally occurring pathway that delays lysosomal degradation of immunoglobulins by cycling them back into circulation, and prolonging their plasma half-life.
12.2 Pharmacodynamics
Hemophilia B is a bleeding disorder characterized by a deficiency of functional coagulation Factor IX (FIX), which leads to a prolonged clotting time in the activated partial thromboplastin time (aPTT) assay, an established in vitro test for the biological activity of Factor IX. Treatment with ALPROLIX shortens the aPTT over the effective dosing period.
12.3 Pharmacokinetics
The pharmacokinetics (PK) of ALPROLIX (rFIXFc) were evaluated in 22 subjects following a 10 minute intravenous infusion of a single dose of 50 IU/kg. The PK parameters (Table 4) were based on plasma FIX activity measured by the one-stage clotting assay. Blood samples for PK analysis were collected prior to dosing and at 11 time points up to 240 hours (10 days) after dosing. The PK data demonstrate that ALPROLIX has a prolonged circulating half-life.
|
Abbreviations: IR = incremental recovery; AUCinf = area under the FIX activity time curve; T1/2 = elimination half-life; MRT = mean residence time; CL = body weight adjusted clearance; Vss = body weight adjusted volume of distribution at steady-state, Time to 1% FIX activity = estimated time in days after dose when FIX activity has declined to approximately 1 IU/dL above baseline |
|
| PK Parameters | rFIXFc (50 IU/kg) (N = 22) |
| Cmax (IU/dL) | 46.04 (68.3%) |
| AUCinf (h*IU/dL) | 1619.1 (26.1%) |
| CL (mL/kg/h) | 3.304 (28.4%) |
| Vss (mL/kg) | 327.0 (28.2%) |
| Terminal T1/2 (h) | 86.52 (37.2%) |
| MRT (h) | 101.96 (29.7%) |
| IR (IU/dL per IU/kg) | 1.0154 (58.7%) |
| Time to 1% FIX activity (d) | 11.489 (23.8%) |
The ALPROLIX PK profile was stable over repeated dosing as shown by comparable PK parameters at Week 26.
The PK parameters following a 100 IU/kg dose of ALPROLIX were evaluated in a subset of 27 subjects. For this subset, the mean drug clearance (CL) was 2.65 (21.7%) mL/kg/h, the maximum activity (Cmax) was 99.89 IU/dL (20.1%) and the time to 1% FIX activity was 15.8 days (21.3%).
Pediatric and Adolescent Pharmacokinetics
Pharmacokinetic (PK) parameters of ALPROLIX (rFIXFc) were determined for adolescents (12 to 17 years of age) and children (2 to 11 years of age) in open-label, multi-center studies of previously treated patients [see Use in Specific Populations (8.4)].
Pharmacokinetic parameters were evaluated following a 10 minute intravenous infusion in 11 evaluable adolescents who received a single dose of ALPROLIX. PK samples were collected prior to dosing and at multiple time points up to 336 hours (14 days) after dosing. In a separate study, PK parameters were evaluated following a 10 minute intravenous infusion in 18 evaluable children (2 to 11 years of age) who received a single dose of ALPROLIX. PK samples were collected prior to dosing and at multiple time points up to 168 hours (7 days) after dosing. PK parameters for ALPROLIX were estimated based on the plasma FIX activity over time profile.
Table 5 presents the PK parameters calculated from the pediatric data of 29 subjects 2 to 17 years of age. Compared to adults, incremental recovery appeared to be lower and body weight- adjusted clearance appeared to be higher in children under 12 years of age. This may result in a need for per kg body weight dose and interval adjustments in children under 12 years of age. [see Use in Specific Populations (8.4)]
|
1PK parameters are presented in arithmetic mean (CV%) |
|||
|
Abbreviations: IR = incremental recovery; AUC = area under the FIX activity time curve; T1/2 = elimination half-life; MRT = mean residence time; CL = body weight adjusted clearance; Vss = body weight adjusted volume of distribution at steady-state |
|||
| PK parameters1 | 2 to 5 years
(N = 5) | 6 to 11 years
(N = 13) | 12 to 17 years
(N = 11) |
| IR (IU/dL per IU/kg) | 0.5980 (15.7%) | 0.7422 (29.2%) | 0.8929 (36.4%) |
| AUC/Dose (IU·h/dL per IU/kg) | 23.18 (15.9%) | 29.38 (26.6%) | 30.23 (22.2%) |
| T1/2 (h) | 66.40 (32.1%) | 72.23 (23.1%) | 83.59 (19.1%) |
| MRT (h) | 80.52 (22.3%) | 84.06 (18.6%) | 95.13 (19.4%) |
| CL (mL/h/kg) | 4.406 (16.8%) | 3.613 (25.1%) | 3.483 (25.6%) |
| Vss (mL/kg) | 349.0 (19.2%) | 303.0 (28.5%) | 326.0 (24.9%) |
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies in animals to evaluate the carcinogenic potential of ALPROLIX, or studies to determine the effects of ALPROLIX on genotoxicity or fertility have not been performed. An assessment of the carcinogenic potential of ALPROLIX was completed and no carcinogenic risk from product use has been identified.
14 CLINICAL STUDIES
The safety and efficacy of ALPROLIX was evaluated in a multi-center, prospective, open-label clinical trial that compared each of two prophylactic treatment regimens (fixed weekly and individualized interval) to episodic (on-demand) treatment; determined hemostatic efficacy in the treatment of bleeding episodes; and determined hemostatic efficacy during perioperative management of subjects undergoing major surgical procedures. A total of 123 previously treated patients (PTPs) with severe hemophilia B (≤ 2% endogenous FIX activity), ages 12-71, were followed for up to 77 weeks.
Sixty three subjects in the fixed weekly interval arm received ALPROLIX for routine prophylaxis starting at an initial dose of 50 IU/kg. The dose was adjusted to maintain FIX trough level between 1% and 3% above baseline or higher, as clinically indicated to prevent bleeding. Fifty subjects required at least one dose adjustment and the median number of dose adjustments was one. The overall median dose on study was 45.2 IU/kg (interquartile range: 38.1, 53.7). The median weekly dose during the last 6 months on study in 58 subjects who were on study for at least 9 months was 40.7 IU/kg (interquartile range: 32.3, 54.1).
Twenty nine subjects in the individualized interval arm received ALPROLIX for routine prophylaxis at a dose of 100 IU/kg every 10 days, with the interval adjusted to maintain FIX trough level between 1% and 3% above baseline or higher, as clinically indicated to prevent bleeding. The overall median interval on study was 12.5 days (interquartile range: 10.4, 13.4). The median interval during the last 6 months in 26 subjects who were on study for at least 9 months was 13.8 days (interquartile range: 10.5, 14.0).
Twenty seven subjects received ALPROLIX as needed for the treatment of bleeding episodes in the episodic (on-demand) treatment arm.
Twelve subjects received ALPROLIX for perioperative management in 14 major surgical procedures. Major surgery was defined as any surgical procedure with or without general anesthesia in which a major body cavity was penetrated and exposed, or a substantial impairment of physical or physiological functions was produced. Four subjects in this arm did not participate in the other arms.
On Demand Treatment and Control of Bleeding Episodes
A total of 636 bleeding events were observed by 114 subjects in the fixed weekly interval prophylaxis, individualized interval prophylaxis, and the episodic (on-demand) arms. The median total dose to treat a bleeding episode was 46.99 IU/kg (interquartile range: 33.33, 62.50). Assessment of response to each injection was recorded by subjects at 8-12 hours after treatment. Efficacy in control of bleeding episodes is summarized in Table 6.
| New Bleeding Episodes | (n=636) |
|---|---|
|
* Excellent: abrupt pain relief and/or improvement in signs of bleeding; Good: definite pain relief and/or improvement in signs of bleeding but possibly requiring another injection in 1-2 days; Moderate: probable or slight beneficial effect and requiring more than one injection; No response: no improvement, or worsening. Response evaluated at approximately 8 hours after treatment. |
|
| Number of injections to treat bleeding episodes | |
| 1 injection | 575 (90.4%) |
| 2 injections | 44 (6.9%) |
| 3 injections | 17 (2.7%) |
| Response* to first injection | (n=613) |
| Excellent or good | 513 (83.7%) |
| Moderate | 90 (14.7%) |
| No response | 10 (1.6%) |
Routine Prophylaxis
Using a negative binomial model, a reduction in annualized bleeding rate (ABR) of 83% (76-89%) for subjects in the fixed weekly interval arm and a reduction of 87% (80-92%) for subjects in the individualized interval arm compared to the episodic (on-demand) treatment arm was observed.
The median duration of treatment on study was 51.4 weeks (range <1-77). A comparison of the ABRs in subjects evaluable for efficacy is summarized in
|
* IQR=interquartile range |
|||
|
Five subjects (2 in prophylaxis fixed weekly interval arm, 3 in prophylaxis individualized interval arm) did not have sufficient data to be included in the efficacy analysis. |
|||
| Bleeding Episode Etiology | Prophylaxis Fixed Weekly Interval (N=61) | Prophylaxis Individualized Interval (N=26) | Episodic (On Demand) (N=27) |
| Overall ABR
(IQR)* | 2.95 (1.01, 4.35) | 1.38 (0.00, 3.43) | 17.69 (10.77, 23.24) |
| Spontaneous ABR (IQR)* | 1.04 (0.00, 2.19) | 0.88 (0.00, 2.30) | 11.78 (2.62, 19.78) |
Perioperative Management
Fourteen major surgical procedures were performed in 12 subjects, including 5 knee replacements, abdominal surgery and a complex dental procedure. Perioperative Factor IX replacement with ALPROLIX was by bolus infusion only. The safety of continuous infusion was not evaluated. Hemostasis was assessed by the investigator at 24 hours after surgery using a 4-point scale of excellent, good, fair, and none. The hemostatic response was rated as excellent (n=13) or good (n=1) in 100% of major surgeries. There were an additional 15 minor surgical procedures in 13 subjects. There was no clinical evidence of thrombotic complications in any of the subjects.
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
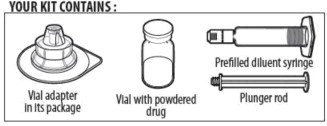
ALPROLIX is supplied as a kit comprising:
- one single-use rFIXFc vial,
- one pre-filled syringe containing 5 mL diluent and sealed with a plunger stopper and tip-cap, and
- one sterile vial adapter (reconstitution device).
ALPROLIX vials are available in 250, 500, 1000, 2000, 3000, or 4000 IU nominal dosage strengths. The actual Factor IX potency, expressed in IU is stated on every rFIXFc vial and kit carton label. Components used in the packaging of ALPROLIX do not contain natural rubber latex.
| Strength | Kit NDC Number |
|---|---|
| 250 IU | 64406-966-01 |
| 500 IU | 64406-911-01 |
| 1000 IU | 64406-922-01 |
| 2000 IU | 64406-933-01 |
| 3000 IU | 64406-944-01 |
| 4000 IU | 64406-977-01 |
Storage and Handling
- Store ALPROLIX in the original package in order to protect it from light.
- Store ALPROLIX at 2°C to 8°C (36°F to 46°F).
- If stored at room temperature, do not to exceed 30°C (86°F) for a single 6 month period. On the carton, record the date when the product was removed from refrigeration. Use the product before the end of this 6 month period or discard it. Do not place the product back into refrigeration after warming to room temperature.
- Do not freeze. Freezing will damage the pre-filled diluent syringe.
- Do not use product or diluent after the expiration date printed on the carton, vial or syringe.
- Reconstituted product may be stored at room temperature, not to exceed 30°C (86°F) for no longer than 3 hours. Protect from direct sunlight. Discard any product not used within 3 hours after reconstitution.
17 PATIENT COUNSELING INFORMATION
- Advise patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
- Advise patients to report any adverse reactions or problems following ALPROLIX administration to their physician or healthcare provider.
- Advise patients to contact their healthcare provider or treatment facility for further treatment and/or assessment if they experience a lack of a clinical response to Factor IX therapy, as this may indicate the development of an inhibitor.
- Inform patients of the early signs of hypersensitivity reactions (including hives, chest tightness, wheezing, difficulty breathing and swelling of the face) and anaphylaxis. Instruct patients to discontinue use of the product and contact their healthcare provider if these symptoms occur.
- Advise patients to contact their healthcare provider or go to the emergency department right away if a thromboembolic event occurs.
Manufactured by
Biogen Inc.
Cambridge, MA 02142 USA
U.S. License #1697
© Bioverativ 2014-2017. All rights reserved.
Patient Information
ALPROLIX® /all' prō liks /
[Coagulation Factor IX (Recombinant), Fc Fusion Protein]
Please read this Patient Information carefully before using ALPROLIX and each time you get a refill, as there may be new information. This Patient Information does not take the place of talking with your healthcare provider about your medical condition or your treatment.
What is ALPROLIX?
ALPROLIX is an injectable medicine that is used to help control and prevent bleeding in people with hemophilia B. Hemophilia B is also called congenital Factor IX deficiency.
Your healthcare provider may give you ALPROLIX when you have surgery.
Who should not use ALPROLIX?
You should not use ALPROLIX if you are allergic to ALPROLIX or any of the other ingredients in ALPROLIX. Tell your healthcare provider if you have had an allergic reaction to any Factor IX product prior to using ALPROLIX.
What should I tell my healthcare provider before using ALPROLIX?
Tell your healthcare provider about all of the medicines you take, including all prescription and non-prescription medicines, such as over-the-counter medicines, supplements, or herbal medicines.
Tell your doctor about all of your medical conditions, including if you:
- are pregnant or planning to become pregnant. It is not known if ALPROLIX may harm your unborn baby.
- are breastfeeding. It is not known if ALPROLIX passes into breast milk or if it can harm your baby.
- have been told that you have inhibitors to Factor IX (because ALPROLIX may not work for you).
How should I use ALPROLIX?
ALPROLIX should be administered as ordered by your healthcare provider. You should be trained on how to do infusions by your healthcare provider. Many people with hemophilia B learn to infuse their ALPROLIX by themselves or with the help of a family member.
See the Instructions for Use for directions on infusing ALPROLIX. The steps in the Instructions for Use are general guidelines for using ALPROLIX. Always follow any specific instructions from your healthcare provider. If you are unsure of the procedure, please ask your healthcare provider. Do not use ALPROLIX as a continuous intravenous infusion.
Contact your healthcare provider immediately if bleeding is not controlled after using ALPROLIX.
What are the possible side effects of ALPROLIX?
Common side effects of ALPROLIX include headache and abnormal sensation in the mouth.
Allergic reactions may occur. Call your healthcare provider or get emergency treatment right away if you have any of the following symptoms: hives, chest tightness, wheezing, difficulty breathing, or swelling of the face.
ALPROLIX may increase the risk of forming abnormal blood clots in your body, especially if you have risk factors for developing blood clots. Call your healthcare provider or seek emergency care if you have symptoms of a possible abnormal blood clot, which may include: chest pain, difficulty breathing, unexpected swelling of an arm or leg with or without pain or tenderness.
Your body can also make antibodies called, “inhibitors,” against ALPROLIX, which may stop ALPROLIX from working properly. Your healthcare provider may need to test your blood for inhibitors from time to time.
These are not all the possible side effects of ALPROLIX.
Talk to your healthcare provider about any side effect that bothers you or that does not go away.
How should I store ALPROLIX?
Store ALPROLIX vials at 2°C to 8°C (36°F to 46°F). Do not freeze.
ALPROLIX vials may also be stored at room temperature up to 30°C (86°F) for a single 6 month period.
If you choose to store ALPROLIX at room temperature:
- Note on the carton the date on which the product was removed from refrigeration.
- Use the product before the end of this 6 month period or discard it.
- Do not return the product to the refrigerator.
Do not use product or diluent after the expiration date printed on the carton, vial or syringe.
After Reconstitution:
- Use the reconstituted product as soon as possible; however, you may store the reconstituted product at room temperature up to 30°C (86°F) for up to 3 hours. Protect the reconstituted product from direct sunlight. Discard any product not used within 3 hours after reconstitution.
- Do not use ALPROLIX if the reconstituted solution is cloudy, contains particles or is not colorless.
What else should I know about ALPROLIX?
Medicines are sometimes prescribed for purposes other than those listed here. Do not use ALPROLIX for a condition for which it was not prescribed. Do not share ALPROLIX with other people, even if they have the same symptoms that you have.
Manufactured by
Biogen Inc.
Cambridge, MA 02142 USA
U.S. License #1697
© Bioverativ 2014-2017. All rights reserved.
ALPROLIX™
Coagulation Factor IX (Recombinant), Fc Fusion Protein
INSTRUCTIONS FOR USE
Read the Instructions for Use before you start using ALPROLIX™ and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or your treatment.
Your healthcare provider should show you or your caregiver how to reconstitute and administer ALPROLIX™ the first time ALPROLIX™ is used.
Check the expiration date on the ALPROLIX™ kit.
Do notuse the product if past the expiration date.
Allow the ALPROLIX™ vial and the diluent to come to room temperature.
Do notuse external heat sources such as putting the vial and/or diluent in hot water.
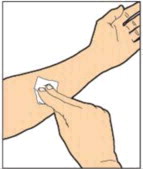
Find a clean, flat work surface and collect all the supplies you will need to reconstitute and administer ALPROLIX™.
Wash your hands with soap and water. Aseptic technique (clean and germ free) should be used.
YOUR KIT CONTAINS:
RECONSTITUTION
POOLING
POOLING is the process of combining two or more reconstituted vials into a larger syringe (not into the diluent syringe) prior to intravenous administration.
If you are using two or more vials, follow these pooling steps.
Be sure to leave the vial adapter attached to the vial as you will need it for attaching a large luer lock syringe.
Do notdetach the diluent syringe or the large luer syringe until you are ready to attach the large luer lock syringe to the next vial (with vial adapter attached).
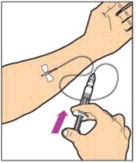
ADMINISTRATION (Intravenous Injection)
ALPROLIX™ is administered by intravenous infusion after reconstitution of the drug powder with the diluent.
Your healthcare provider should teach you how to infuse ALPROLIX™. Once you have been taught to self-infuse, you can follow these instructions.
Do notadminister reconstituted ALPROLIX™ if it contains particulate matter, is discolored, or is cloudy.
STORAGE CONDITIONS - PRODUCT KIT
Keep refrigerated until use.
Keep away from direct sunlight.
STORAGE CONDITIONS - RECONSTITUTED
ALPROLIX™ should be administered within 3 hours after reconstitution.
Do not refrigerate after reconstitution.
Keep away from direct sunlight
Revised March 2014
Manufactured by:
Biogen Idec, Inc.
14 Cambridge Center, Cambridge, MA 02142 USA
U.S. License #1697
©2014 Biogen Idec Inc. All rights reserved.
For more information go to www.ALPROLIX.com or call 1-855-692-5776
Principal Display Panel - 250 IU Nominal Carton Label
NDC 64406-966-01
One single-use vial
with 5 mL prefilled
diluent syringe
ALPROLIX™
Coagulation Factor IX
(Recombinant), Fc Fusion Protein
For Intravenous Administration
Rx Only
Manufactured by:
Biogen Inc.
Cambridge, MA 02142
US License Number 1697
Principal Display Panel - 250 IU Vial Label
ALPROLIX™ NDC 64406-952-01
Coagulation Factor IX (Recombinant), Fc Fusion Protein
For Intravenous Administration Rx Only
Store in a refrigerator
at 2°C to 8°/36°F to 46°F.
RECONSTITUTE ONLY
WITH DILUENT PROVIDED
Dosage & Administration:
See Package Insert
No preservatives
Manufactured by:
Biogen Inc.
Cambridge, MA 02142
US License Number 1697
Principal Display Panel - 500 IU Nominal Carton Label
NDC 64406-911-01
One single-use vial
with 5 mL prefilled
diluent syringe
ALPROLIX™
Coagulation Factor IX
(Recombinant), Fc Fusion Protein
For Intravenous Administration
Rx Only
Manufactured by:
Biogen Inc.
Cambridge, MA 02142
US License Number 1697
Principal Display Panel - 500 IU Vial Label
ALPROLIX™ NDC 64406-953-09
Coagulation Factor IX (Recombinant), Fc Fusion Protein
For Intravenous Administration Rx Only
Store in a refrigerator
at 2°C to 8°/36°F to 46°F.
RECONSTITUTE ONLY
WITH DILUENT PROVIDED
Dosage & Administration:
See Package Insert
No preservatives
Manufactured by:
Biogen Inc.
Cambridge, MA 02142
US License Number 1697
Principal Display Panel - 1000 IU Nominal Carton Label
NDC 64406-922-01
One single-use vial
with 5 mL prefilled
diluent syringe
ALPROLIX™
Coagulation Factor IX
(Recombinant), Fc Fusion Protein
For Intravenous Administration
Rx Only
Manufactured by:
Biogen Inc.
Cambridge, MA 02142
US License Number 1697
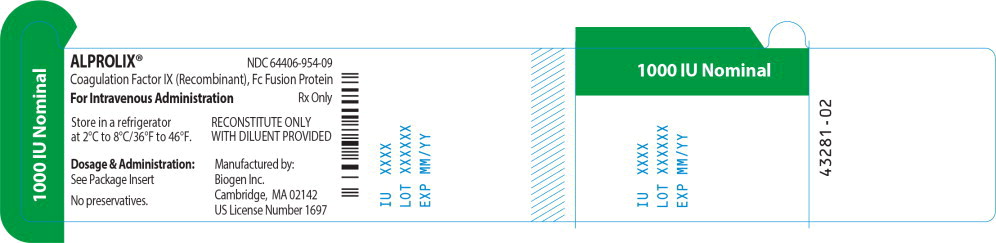
Principal Display Panel - 1000 IU Vial Label
ALPROLIX™ NDC 64406-954-09
Coagulation Factor IX (Recombinant), Fc Fusion Protein
For Intravenous Administration Rx Only
Store in a refrigerator
at 2°C to 8°/36°F to 46°F.
RECONSTITUTE ONLY
WITH DILUENT PROVIDED
Dosage & Administration:
See Package Insert
No preservatives
Manufactured by:
Biogen Inc.
Cambridge, MA 02142
US License Number 1697
Principal Display Panel - 2000 IU Nominal Carton Label
NDC 64406-933-01
One single-use vial
with 5 mL prefilled
diluent syringe
ALPROLIX™
Coagulation Factor IX
(Recombinant), Fc Fusion Protein
For Intravenous Administration
Rx Only
Manufactured by:
Biogen Inc.
Cambridge, MA 02142
US License Number 1697
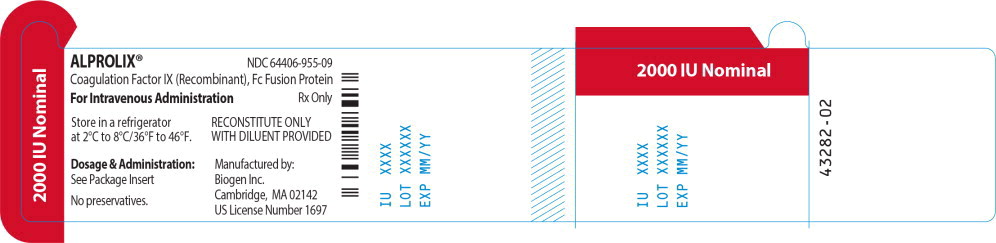
Principal Display Panel - 2000 IU Vial Label
ALPROLIX™ NDC 64406-955-09
Coagulation Factor IX (Recombinant), Fc Fusion Protein
For Intravenous Administration Rx Only
Store in a refrigerator
at 2°C to 8°/36°F to 46°F.
RECONSTITUTE ONLY
WITH DILUENT PROVIDED
Dosage & Administration:
See Package Insert
No preservatives
Manufactured by:
Biogen Inc.
Cambridge, MA 02142
US License Number 1697
Principal Display Panel - 3000 IU Nominal Carton Label
NDC 64406-944-01
One single-use vial
with 5 mL prefilled
diluent syringe
ALPROLIX™
Coagulation Factor IX
(Recombinant), Fc Fusion Protein
For Intravenous Administration
Rx Only
Manufactured by:
Biogen Inc.
Cambridge, MA 02142
US License Number 1697
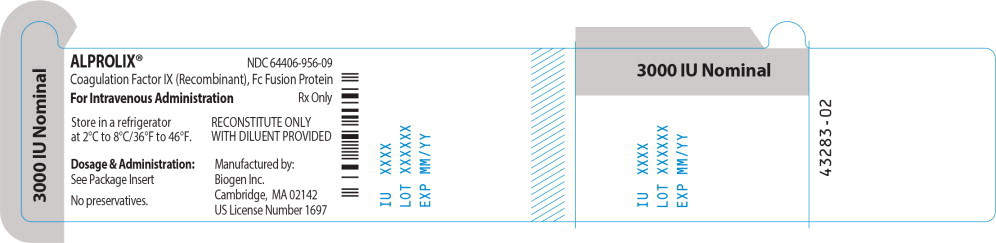
Principal Display Panel - 3000 IU Vial Label
ALPROLIX™ NDC 64406-956-09
Coagulation Factor IX (Recombinant), Fc Fusion Protein
For Intravenous Administration Rx Only
Store in a refrigerator
at 2°C to 8°/36°F to 46°F.
RECONSTITUTE ONLY
WITH DILUENT PROVIDED
Dosage & Administration:
See Package Insert
No preservatives
Manufactured by:
Biogen Inc.
Cambridge, MA 02142
US License Number 1697
Principal Display Panel - 4000 IU Nominal Carton Label
NDC 64406-977-01
One single-use vial
with 5 mL prefilled
diluent syringe
ALPROLIX™
Coagulation Factor IX
(Recombinant), Fc Fusion Protein
For Intravenous Administration
Rx Only
Manufactured by:
Biogen Inc.
Cambridge, MA 02142
US License Number 1697
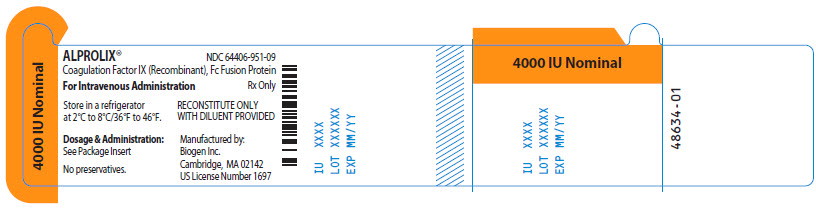
Principal Display Panel - 4000 IU Vial Label
ALPROLIX™ NDC 64406-951-09
Coagulation Factor IX (Recombinant), Fc Fusion Protein
For Intravenous Administration Rx Only
Store in a refrigerator
at 2°C to 8°/36°F to 46°F.
RECONSTITUTE ONLY
WITH DILUENT PROVIDED
Dosage & Administration:
See Package Insert
No preservatives
Manufactured by:
Biogen Inc.
Cambridge, MA 02142
US License Number 1697
Principal Display Panel - Diluent Vial Label
Sterile Sodium Chloride
Solution 0.325% 5 mL
Diluent for accompanying product.
For intravenous use.
Single-use. Rx only
Contains no preservatives.
Manufactured for:
Biogen Inc., Cambridge, MA 02142
US License # 1697
Manufactured by:
Vetter Pharma-Fertigung GmbH & Co. KG
88212 Ravensburg, Germany
| ALPROLIX
coagulation factor ix (recombinant), fc fusion protein kit |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| ALPROLIX
coagulation factor ix (recombinant), fc fusion protein kit |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| ALPROLIX
coagulation factor ix (recombinant), fc fusion protein kit |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| ALPROLIX
coagulation factor ix (recombinant), fc fusion protein kit |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| ALPROLIX
coagulation factor ix (recombinant), fc fusion protein kit |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| ALPROLIX
coagulation factor ix (recombinant), fc fusion protein kit |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Biogen Inc. (121376230) |