CLOTRIMAZOLE ATHLETES FOOT- clotrimazole cream
Kaiser Foundation Hospitals
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
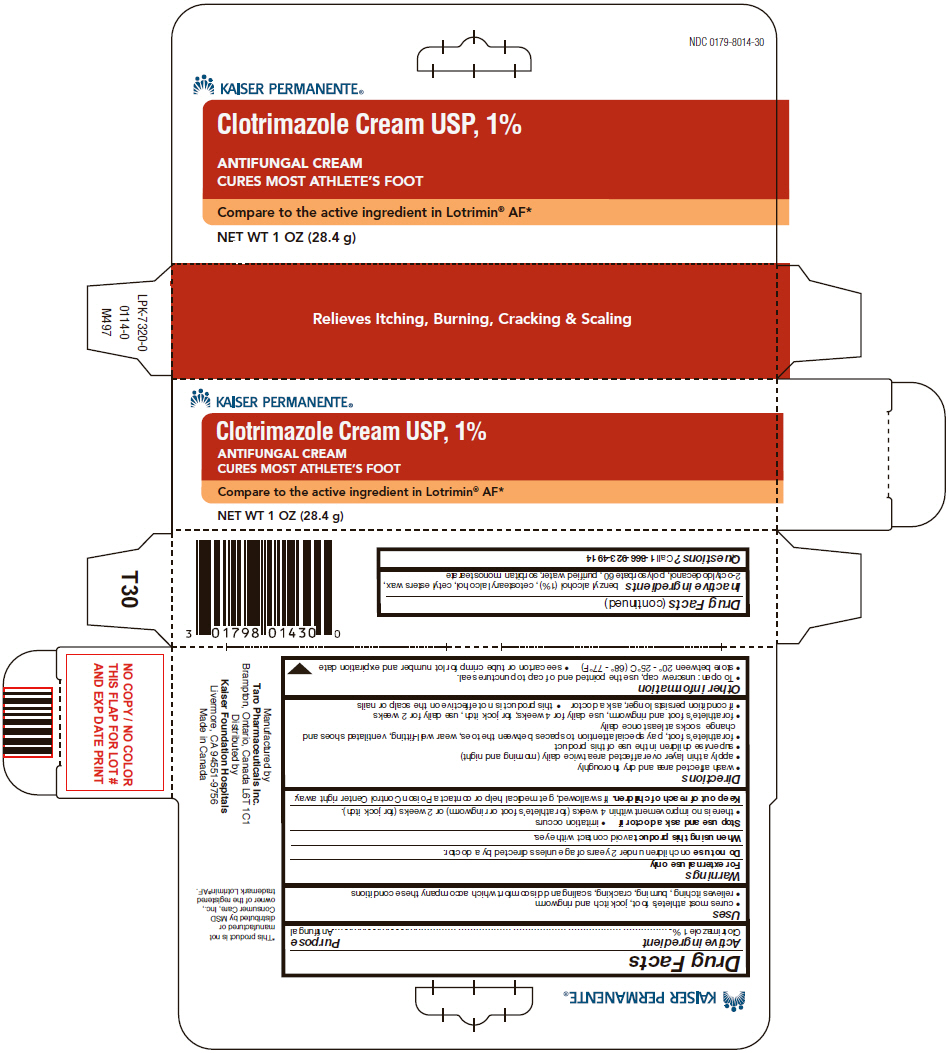
Clotrimazole USP, 1%
Athlete's Foot
Uses
- cures most athlete's foot, jock itch and ringworm
- relieves itching, burning, cracking, scaling and discomfort which accompany these conditions
Warnings
For external use only
Directions
- wash affected area and dry thoroughly
- apply a thin layer over affected area twice daily (morning and night)
- supervise children in the use of this product
- for athlete's foot, pay special attention to spaces between the toes, wear well-fitting, ventilated shoes and change socks at least once daily
- for athlete's foot and ringworm, use daily for 4 weeks; for jock itch, use daily for 2 weeks
- if condition persists longer, ask a doctor
- this product is not effective on the scalp or nails
Other information
- To open: unscrew cap, use the pointed end of cap to puncture seal.
- store between 20° - 25°C (68° - 77°F)
- see carton or tube crimp for lot number and expiration date
Inactive ingredients
benzyl alcohol (1%), cetostearyl alcohol, cetyl esters wax, 2-octyldodecanol, polysorbate 60, purified water, sorbitan monostearate
| CLOTRIMAZOLE
ATHLETES FOOT
clotrimazole cream |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Kaiser Foundation Hospitals (053052619) |
| Registrant - Taro Pharmaceuticals U.S.A., Inc. (145186370) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Taro Pharmaceuticals Inc. | 206263295 | manufacture(0179-8014) | |
Revised: 11/2018
Document Id: 7ad27cc5-ac46-1c22-e053-2991aa0a663b
Set id: 1aca67ce-73e5-4ed5-89ff-55f3294d5e5e
Version: 3
Effective Time: 20181116
Kaiser Foundation Hospitals