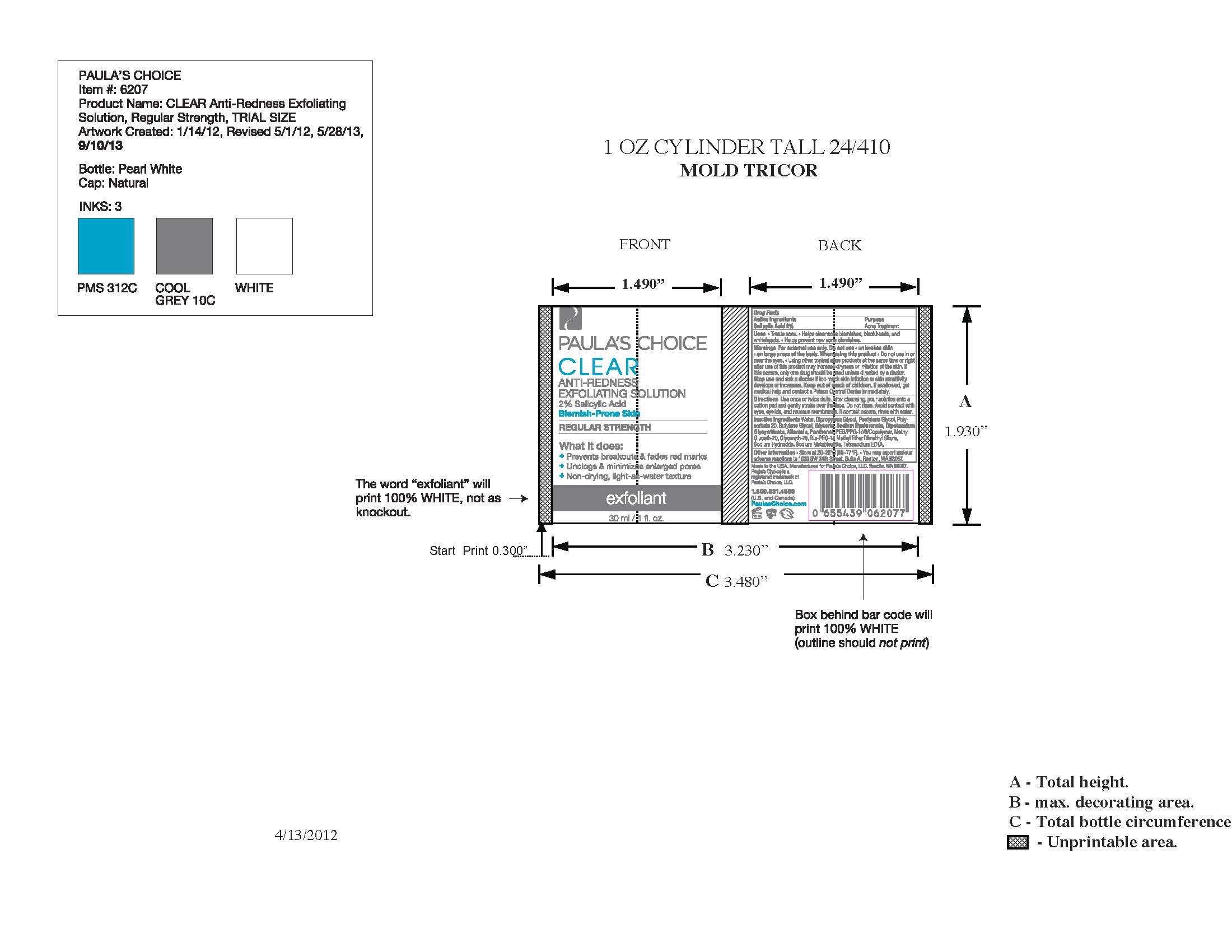
PAULAS CHOICE CLEAR ANTI REDNESS EXFOLIATING SOLUTION- salicylic acid liquid
Paula's Choice, LLC.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Paulas Choice CLEAR Anti-Redness Exfoliating Solution 2% Salicylic Acid, Regular Strength
After cleansing with Paula's Choice Clear Pore Normalizing Cleanser, soak a large cotton ball with Regular Strength Anti-Redness Exfoliating Solution and gently stroke over the face, paying special attention to acne and blackhead prone areas. Do not rinse. Avoid contact with eyes, eyelids, and mucous membranes. If contact occurs, rinse with water. If excessive dryness or peeling occurs, reduce application to once per day or once every other day.
When using this product
· Do not use in or near the eyes
· Using other topical acne products at the same time or right after use of this product may increase dryness or irritation of the skin. If this occurs, only one drug should be used unless directed by a doctor.
Keep out of reach of children. If swallowed, get medical help and contact a Poison Control Center immediately.
- Store at 20-25ºC (68-77ºF).
- You may report serious adverse reactions to 1030 SW 34th Street, Suite A, Renton, WA 98057
Water, Dipropylene Glycol, Pentylene Glycol, Polysorbate 20, Butylene Glycol, Glycerin, Sodium Hyaluronate, Dipotassium Glycyrrhizate, Allantoin. Panthenol, PEG/PPG-17/6 Copolymer, Methyl Gluceth-20, Glycereth-26, Bis-PEG-18 Methyl Ether Dimethyl Silane, Sodium Hydroxide, Sodium Metabisulfite, Tetrasodium EDTA
| PAULAS CHOICE CLEAR ANTI REDNESS EXFOLIATING SOLUTION
salicylic acid liquid |
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
| Labeler - Paula's Choice, LLC. (029583981) |