PFIZER-BIONTECH COVID-19 VACCINE- bnt162b2 injection, suspension
Pfizer Manufacturing Belgium NV
Reference Label Set Id: 8003e2b9-ea36-4275-b0ba-673460fd6305
----------
PFIZER-BIONTECH COVID-19 VACCINE
FACT SHEET FOR HEALTHCARE PROVIDERS ADMINISTERING VACCINE (VACCINATION PROVIDERS)
EMERGENCY USE AUTHORIZATION (EUA) OF THE PFIZER-BIONTECH COVID-19 VACCINE AND THE PFIZER-BIONTECH COVID-19 VACCINE, BIVALENT (ORIGINAL AND OMICRON BA.4/BA.5) TO PREVENT CORONAVIRUS DISEASE 2019 (COVID-19)
|
FOR 6 MONTHS THROUGH 4 YEARS OF AGE
DILUTE BEFORE USE |
The U.S. Food and Drug Administration (FDA) has issued an Emergency Use Authorization (EUA) to permit the emergency use of the unapproved products, Pfizer-BioNTech COVID-19 Vaccine1 and Pfizer-BioNTech COVID-19 Vaccine, Bivalent (Original and Omicron BA.4/BA.5)2, for active immunization to prevent COVID-19 in individuals 6 months of age and older.
The Pfizer-BioNTech COVID-19 Vaccine, Bivalent (Original and Omicron BA.4/BA.5) is hereafter referred to as Pfizer-BioNTech COVID-19 Vaccine, Bivalent.
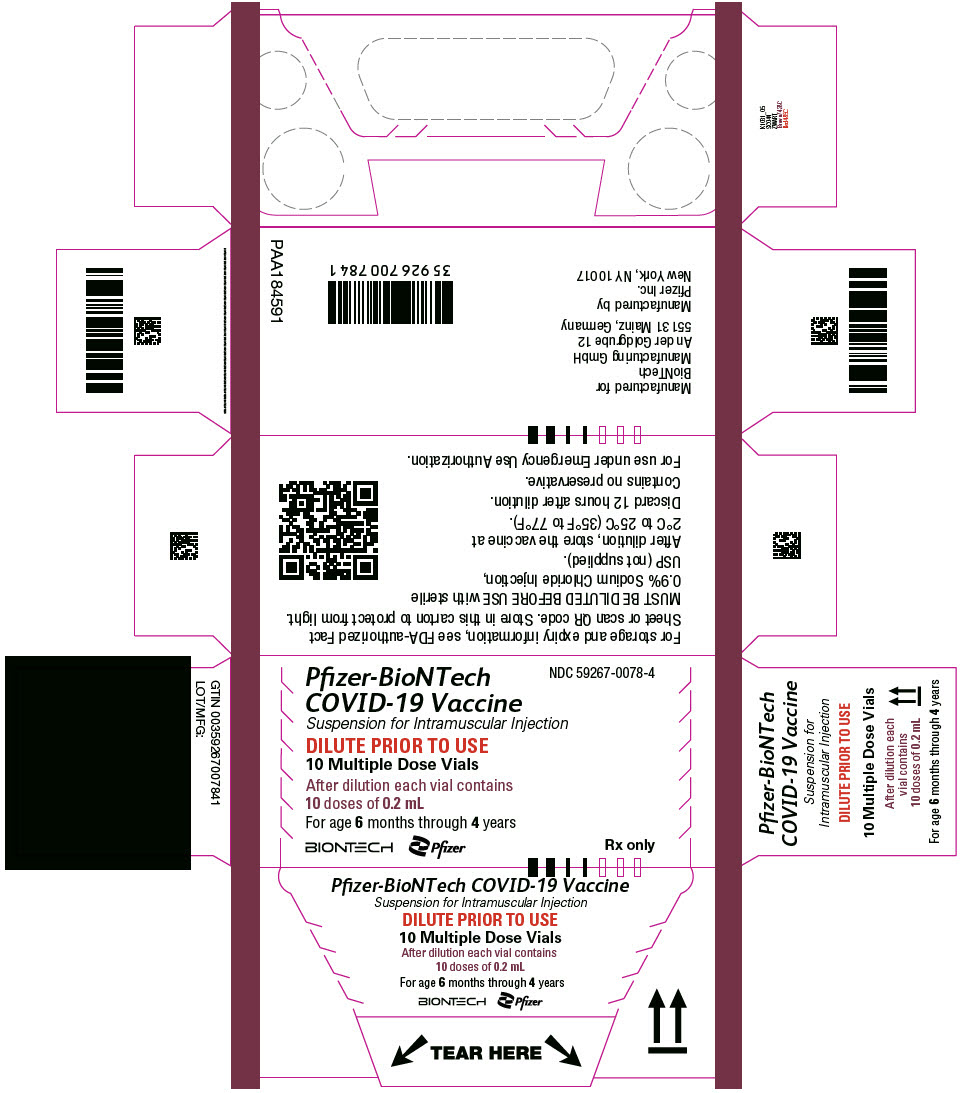
The Pfizer-BioNTech COVID-19 Vaccine and the Pfizer-BioNTech COVID-19 Vaccine, Bivalent for use in individuals 6 months through 4 years of age are supplied in multiple dose vials with maroon caps and labels with maroon borders.
Primary Series
Pfizer-BioNTech COVID-19 Vaccine and Pfizer-BioNTech COVID-19 Vaccine, Bivalent are authorized for use in individuals 6 months through 4 years of age to provide a 3-dose primary series as follows:
- Dose 1: Pfizer-BioNTech COVID-19 Vaccine
- Dose 2: Pfizer-BioNTech COVID-19 Vaccine
- Dose 3: Pfizer-BioNTech COVID-19 Vaccine, Bivalent
Booster Dose
The Pfizer-BioNTech COVID-19 Vaccine, Bivalent is authorized for use in individuals 6 months through 4 years of age to provide a single booster dose at least 2 months after completion of primary vaccination with 3 doses of the Pfizer-BioNTech COVID-19 Vaccine3.
This Fact Sheet pertains only to Pfizer-BioNTech COVID-19 Vaccine and Pfizer-BioNTech COVID-19 Vaccine, Bivalent supplied in multiple dose vials with maroon caps and labels with maroon borders, which MUST BE DILUTED PRIOR TO USE.
The Pfizer-BioNTech COVID-19 Vaccine vial labels may state "Age 2y to < 5y" or "Age 6m to < 5y" and carton labels may state "For age 2 years to < 5 years" or "For age 6 months to < 5 years". Vials with either printed age range can be used in individuals 6 months through 4 years of age.
Pfizer-BioNTech COVID-19 Vaccine and Pfizer-BioNTech COVID-19, Bivalent which are supplied in multiple dose vials with maroon caps and labels with maroon borders, should not be used in individuals 5 years of age and older because of the potential for vaccine administration errors, including dosing errors.4
SUMMARY OF INSTRUCTIONS FOR COVID-19 VACCINATION PROVIDERS
Vaccination providers enrolled in the federal COVID-19 Vaccination Program must report all vaccine administration errors, all serious adverse events, cases of myocarditis, cases of pericarditis, cases of Multisystem Inflammatory Syndrome (MIS) in adults and children, and cases of COVID-19 that result in hospitalization or death following administration of Pfizer-BioNTech COVID-19 Vaccine or Pfizer-BioNTech COVID-19 Vaccine, Bivalent. See "MANDATORY REQUIREMENTS FOR PFIZER-BIONTECH COVID-19 VACCINE AND PFIZER-BIONTECH COVID-19 VACCINE, BIVALENT ADMINISTRATION UNDER EMERGENCY USE AUTHORIZATION" for reporting requirements.
The Pfizer-BioNTech COVID-19 Vaccine and the Pfizer-BioNTech COVID-19 Vaccine, Bivalent are suspensions for intramuscular injection.
Primary Series
The Pfizer-BioNTech COVID-19 Vaccine and the Pfizer-BioNTech COVID-19 Vaccine, Bivalent for individuals 6 months through 4 years of age are supplied in multiple dose vials with maroon caps and labels with maroon borders and after dilution are administered intramuscularly as a 3-dose (0.2 mL each) primary series as follows:
- Dose 1: Pfizer-BioNTech COVID-19 Vaccine
- Dose 2: Pfizer-BioNTech COVID-19 Vaccine
- Dose 3: Pfizer-BioNTech COVID-19 Vaccine, Bivalent
Dose 1 and Dose 2 (Pfizer-BioNTech COVID-19 Vaccine) are administered 3 weeks apart. Dose 3 (Pfizer-BioNTech COVID-19 Vaccine, Bivalent) is administered at least 8 weeks after Dose 2.
Individuals who will turn from 4 years to 5 years of age between any doses in the primary series5 may receive either:
- •
- a 3-dose primary series comprised of Pfizer-BioNTech COVID-19 Vaccine (supplied in multiple dose vials with maroon caps and labels with maroon borders) for Doses 1 and 2 and Pfizer-BioNTech COVID-19 Vaccine, Bivalent (supplied in multiple dose vials with maroon caps and labels with maroon borders) for Dose 3, or
- •
- a 2-dose primary series with the Pfizer-BioNTech COVID-19 Vaccine authorized for use for individuals 5 through 11 years of age (supplied in multiple dose vials with orange caps and labels with orange borders).
Booster Dose
The Pfizer-BioNTech COVID-19 Vaccine, Bivalent is authorized for use in individuals 6 months through 4 years of age to provide a single booster dose (0.2 mL) at least 2 months after completion of primary vaccination with 3 doses of the Pfizer-BioNTech COVID-19 Vaccine.
See this Fact Sheet for instructions for preparation and administration. This Fact Sheet may have been updated. For the most recent Fact Sheet, please see www.cvdvaccine.com.
For information on clinical trials that are testing the use of the Pfizer-BioNTech COVID-19 Vaccine and the Pfizer-BioNTech COVID-19 Vaccine, Bivalent for active immunization to prevent COVID-19, please see www.clinicaltrials.gov.
DESCRIPTION OF COVID-19
Coronavirus disease 2019 (COVID-19) is an infectious disease caused by the novel coronavirus, SARS-CoV-2, that appeared in late 2019. It is predominantly a respiratory illness that can affect other organs. People with COVID-19 have reported a wide range of symptoms, ranging from mild symptoms to severe illness. Symptoms may appear 2 to 14 days after exposure to the virus. Symptoms may include: fever or chills; cough; shortness of breath; fatigue; muscle or body aches; headache; new loss of taste or smell; sore throat; congestion or runny nose; nausea or vomiting; diarrhea.
DOSAGE AND ADMINISTRATION
The storage, preparation, and administration information in this Fact Sheet apply to the Pfizer-BioNTech COVID-19 Vaccine and the Pfizer-BioNTech COVID-19 Vaccine, Bivalent which are supplied in multiple dose vials with maroon caps and labels with maroon borders and MUST BE DILUTED before use.
| Age Range | Dilution Information | Doses Per Vial After Dilution | Dose Volume |
|---|---|---|---|
|
|||
|
6 months through 4 years* |
Dilute with 2.2 mL sterile 0.9% Sodium Chloride Injection, USP prior to use |
10 |
0.2 mL |
Storage and Handling
During storage, minimize exposure to room light, and avoid exposure to direct sunlight and ultraviolet light.
Do not refreeze thawed vials.
Vial Storage Prior to Use
Cartons of Pfizer-BioNTech COVID-19 Vaccine and Pfizer-BioNTech COVID-19 Vaccine, Bivalent multiple dose vials with maroon caps and labels with maroon borders may arrive frozen at ultra-cold conditions in thermal containers with dry ice.
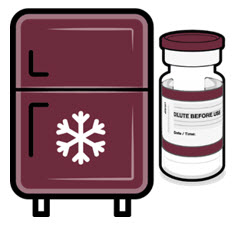
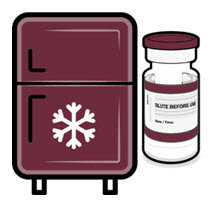
Once received, frozen vials may be immediately transferred to the refrigerator [2°C to 8°C (35°F to 46°F)], thawed and stored for up to 10 weeks. The 10-week refrigerated expiry date should be recorded on the carton at the time of transfer. A carton of 10 vials may take up to 2 hours to thaw at this temperature.
Alternatively, frozen vials may be stored in an ultra-low temperature freezer at -90°C to -60°C (-130°F to -76°F) for up to 18 months from the date of manufacture. Do not store vials at -25°C to -15°C (-13°F to 5°F). Once vials are thawed, they should not be refrozen.
If cartons of Pfizer-BioNTech COVID-19 Vaccine and Pfizer-BioNTech COVID-19 Vaccine, Bivalent multiple dose vials with maroon caps and labels with maroon borders are received at 2°C to 8°C (35°F to 46°F), they should be stored at 2°C to 8°C (35°F to 46°F). Check that the carton has been updated to reflect the 10-week refrigerated expiry date.
Regardless of storage condition, the vaccine should not be used after 18 months from the date of manufacture printed on the vial and cartons. Examples of expiry dates based on 18 months from the date of the manufacture for the Pfizer-BioNTech COVID-19 Vaccine or the Pfizer-BioNTech COVID-19 Vaccine, Bivalent are shown below.
|
Printed Manufacturing Date |
18-Month Expiry Date |
|
|
01/2022 |
|
30-Jun-2023 |
|
02/2022 |
|
31-Jul-2023 |
|
03/2022 |
|
31-Aug-2023 |
|
04/2022 |
|
30-Sep-2023 |
|
05/2022 |
|
31-Oct-2023 |
|
06/2022 |
|
30-Nov-2023 |
Vial Storage During Use
If not previously thawed at 2°C to 8°C (35°F to 46°F), allow vials to thaw at room temperature [up to 25°C (77°F)] for 30 minutes.
Pfizer-BioNTech COVID-19 Vaccine and Pfizer-BioNTech COVID-19 Vaccine, Bivalent multiple dose vials with maroon caps and labels with maroon borders may be stored at room temperature [8°C to 25°C (46°F to 77°F)] for a total of 12 hours prior to dilution.
After dilution, the vial should be held between 2ºC to 25°C (35°F to 77°F). Vials should be discarded 12 hours after dilution.
Pfizer-BioNTech COVID-19 Vaccine vial labels and cartons may state that vials should be discarded 6 hours after the first puncture. The information in this Fact Sheet supersedes the number of hours printed on vial labels and cartons.
Transportation of Vials
If local redistribution is needed, undiluted vials may be transported at -90°C to -60°C (-130°F to -76°F) or at 2°C to 8°C (35°F to 46°F).
Dosing and Schedule
Primary Series
The Pfizer-BioNTech COVID-19 Vaccine and the Pfizer-BioNTech COVID-19 Vaccine, Bivalent for individuals 6 months through 4 years of age is supplied in multiple dose vials with maroon caps and labels with maroon borders and after dilution is administered intramuscularly as a 3-dose (0.2 mL each) primary series as follows:
- Dose 1: Pfizer-BioNTech COVID-19 Vaccine
- Dose 2: Pfizer-BioNTech COVID-19 Vaccine
- Dose 3: Pfizer-BioNTech COVID-19 Vaccine, Bivalent
Dose 1 and Dose 2 (Pfizer-BioNTech COVID-19 Vaccine) are administered 3 weeks apart. Dose 3 (Pfizer-BioNTech COVID-19 Vaccine, Bivalent) is administered at least 8 weeks after Dose 2.
Individuals who will turn from 4 years to 5 years of age between any doses in the primary series may receive either:
- •
- a 3-dose primary series comprised of Pfizer-BioNTech COVID-19 Vaccine (supplied in multiple dose vials with maroon caps and labels with maroon borders) for Doses 1 and 2 and Pfizer-BioNTech COVID-19 Vaccine, Bivalent (supplied in multiple dose vials with maroon caps and labels with maroon borders) for Dose 3, or
- •
- a 2-dose primary series with the Pfizer-BioNTech COVID-19 Vaccine authorized for use for individuals 5 through 11 years of age (supplied in multiple dose vials with orange caps and labels with orange borders).
Booster Dose
The Pfizer-BioNTech COVID-19 Vaccine, Bivalent is authorized for use in individuals 6 months through 4 years of age to provide a single booster dose (0.2 mL) at least 2 months after completion of primary vaccination with 3 doses of the Pfizer-BioNTech COVID-19 Vaccine.
Dose Preparation
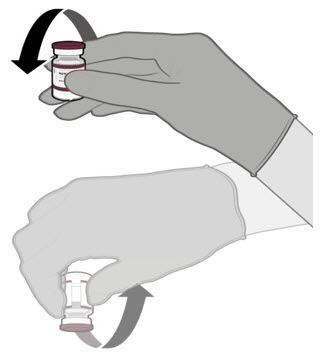
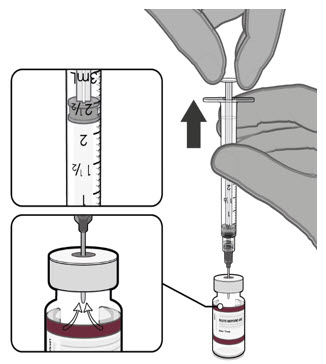
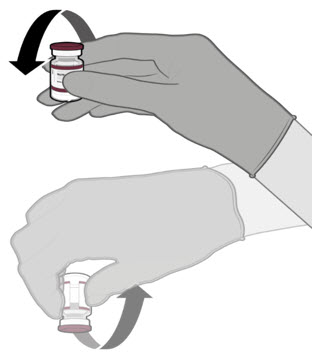
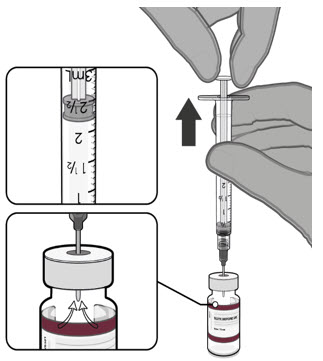
Each vial MUST BE DILUTED before administering the vaccine.
Prior to Dilution
- •
- The Pfizer-BioNTech COVID-19 Vaccine and the Pfizer-BioNTech COVID-19 Vaccine, Bivalent multiple dose vials with maroon caps and labels with maroon borders contain a volume of 0.4 mL, and are supplied as frozen suspensions that do not contain preservative.
- •
- Each vial must be thawed before dilution.
- o
- Vials may be thawed in the refrigerator [2°C to 8°C (35°F to 46°F)] or at room temperature [up to 25°C (77°F)].
- o
- Refer to thawing instructions in the panels below.
Dilution
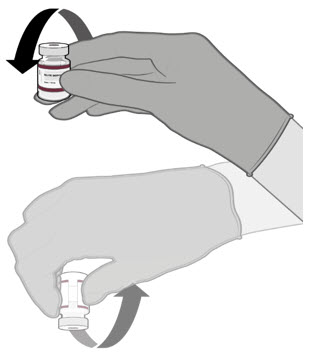
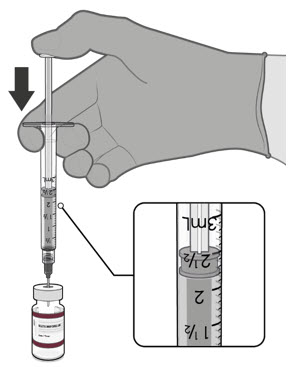
Dilute the vial contents using 2.2 mL of sterile 0.9% Sodium Chloride Injection, USP (not provided) to form the Pfizer-BioNTech COVID-19 Vaccine or the Pfizer-BioNTech COVID-19 Vaccine, Bivalent.
ONLY use sterile 0.9% Sodium Chloride Injection, USP as the diluent. This diluent is not packaged with the vaccine and must be sourced separately. Do not use bacteriostatic 0.9% Sodium Chloride Injection or any other diluent. Do not add more than 2.2 mL of diluent.
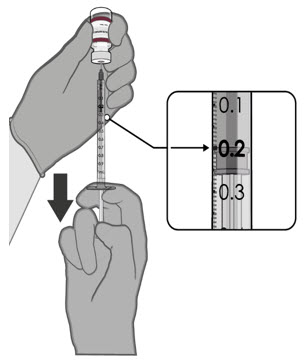
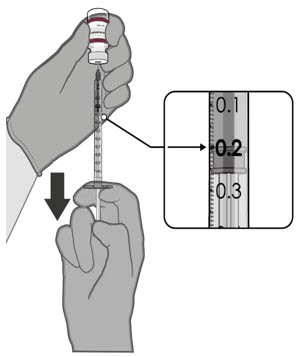
After dilution, 1 vial contains 10 doses of 0.2 mL.
Administration
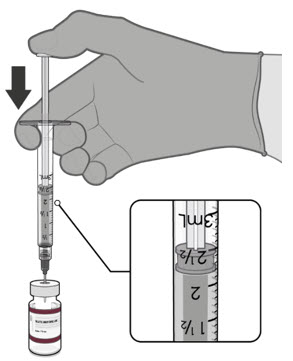
Visually inspect each dose in the dosing syringe prior to administration. The vaccine will be a white to off-white suspension. During the visual inspection,
- •
- verify the final dosing volume of 0.2 mL.
- •
- confirm there are no particulates and that no discoloration is observed.
- •
- do not administer if vaccine is discolored or contains particulate matter.
Administer the Pfizer-BioNTech COVID-19 Vaccine or Pfizer-BioNTech COVID-19 Vaccine, Bivalent intramuscularly.
After dilution, vials of Pfizer-BioNTech COVID-19 Vaccine or Pfizer-BioNTech COVID-19 Vaccine, Bivalent with maroon caps and labels with maroon borders contain 10 doses of 0.2 mL of vaccine. Low dead-volume syringes and/or needles can be used to extract 10 doses from a single vial. If standard syringes and needles are used, there may not be sufficient volume to extract 10 doses from a single vial. Irrespective of the type of syringe and needle:
- •
- Each dose must contain 0.2 mL of vaccine.
- •
- If the amount of vaccine remaining in the vial cannot provide a full dose of 0.2 mL, discard the vial and content.
- •
- Do not pool excess vaccine from multiple vials.
Contraindications
Do not administer Pfizer-BioNTech COVID-19 Vaccine or Pfizer-BioNTech COVID-19 Vaccine, Bivalent to individuals with known history of a severe allergic reaction (e.g., anaphylaxis) to any component of the Pfizer-BioNTech COVID-19 Vaccine (see Full EUA Prescribing Information).
Warnings
Management of Acute Allergic Reactions
Appropriate medical treatment used to manage immediate allergic reactions must be immediately available in the event an acute anaphylactic reaction occurs following administration of Pfizer-BioNTech COVID-19 Vaccine.
Monitor Pfizer-BioNTech COVID-19 Vaccine and Pfizer-BioNTech COVID-19 Vaccine, Bivalent recipients for the occurrence of immediate adverse reactions according to the Centers for Disease Control and Prevention (CDC) guidelines (https://www.cdc.gov/vaccines/covid-19/clinical-considerations/managing-anaphylaxis.html).
Myocarditis and Pericarditis
Postmarketing safety data with Pfizer-BioNTech COVID-19 Vaccine are relevant to Pfizer-BioNTech COVID-19 Vaccine, Bivalent because these vaccines are manufactured using the same process.
Postmarketing data with authorized or approved monovalent mRNA COVID-19 vaccines demonstrate increased risks of myocarditis and pericarditis, particularly within the first week following receipt of the second dose of a 2-dose primary series or the first booster dose (third dose), with most booster doses likely administered at least 5 months after completing primary vaccination. For the Pfizer-BioNTech COVID-19 Vaccine, the observed risk is highest in males 12 through 17 years of age. Although some cases required intensive care support, available data from short-term follow-up suggest that most individuals have had resolution of symptoms with conservative management. Information is not yet available about potential long-term sequelae. The CDC has published considerations related to myocarditis and pericarditis after vaccination, including for vaccination of individuals with a history of myocarditis or pericarditis (https://www.cdc.gov/vaccines/covid-19/clinical-considerations/myocarditis.html).
Syncope
Syncope (fainting) may occur in association with administration of injectable vaccines, in particular in adolescents. Procedures should be in place to avoid injury from fainting.
Altered Immunocompetence
Immunocompromised persons, including individuals receiving immunosuppressant therapy, may have a diminished immune response to the Pfizer-BioNTech COVID-19 Vaccine or the Pfizer-BioNTech COVID-19 Vaccine, Bivalent.
Limitation of Effectiveness
The Pfizer-BioNTech COVID-19 Vaccine and the Pfizer-BioNTech COVID-19 Vaccine, Bivalent may not protect all vaccine recipients.
Adverse Reactions
Adverse Reactions in Clinical Trials
The safety of Pfizer-BioNTech COVID-19 Vaccine in individuals 6 months through 4 years of age is based on:
- •
- safety data from a clinical study which evaluated a 3-dose primary series of Pfizer-BioNTech COVID-19 Vaccine in individuals 6 months through 4 years of age,
- •
- safety data from clinical studies which evaluated a 2-dose primary series of Pfizer-BioNTech COVID-19 Vaccine in individuals 5 years of age and older, and
- •
- postmarketing safety data with the Pfizer-BioNTech COVID-19 Vaccine.
The safety of the Pfizer-BioNTech COVID-19 Vaccine, Bivalent in individuals 6 months through 4 years of age is based on:
- •
- safety data from clinical studies which evaluated a booster dose of Pfizer-BioNTech Vaccine, Bivalent (Original and Omicron BA.4/BA.5) in individuals 6 months of age and older,
- •
- safety data from a clinical study which evaluated a booster dose of Pfizer-BioNTech's bivalent COVID-19 vaccine (Original and Omicron BA.1), not authorized or approved, hereafter referred to as bivalent vaccine (Original and Omicron BA.1) in individuals greater than 55 years of age,
- o
- The safety data accrued with the bivalent vaccine (Original and Omicron BA.1) and with the Pfizer-BioNTech COVID-19 Vaccine are relevant to the Pfizer-BioNTech COVID-19 Vaccine, Bivalent because these vaccines are manufactured using the same process.
- •
- safety data from clinical trials which evaluated primary vaccination with the Pfizer-BioNTech COVID-19 Vaccine in individuals 6 months of age and older and
- •
- safety data from clinical trials which evaluated booster vaccination in individuals 5 years of age and older with the Pfizer-BioNTech COVID-19 Vaccine (previously, but no longer, authorized), and
- •
- postmarketing safety data with the Pfizer-BioNTech COVID-19 Vaccine and the Pfizer-BioNTech COVID-19 Vaccine, Bivalent.
Adverse reactions in participants 6 through 23 months of age following administration of the Pfizer-BioNTech COVID-19 Vaccine and the Pfizer-BioNTech COVID-19 Vaccine, Bivalent included irritability, drowsiness, decreased appetite, tenderness at the injection site, injection site redness, fever, injection site swelling, fatigue, and lymphadenopathy (see Full EUA Prescribing Information).
Adverse reactions in participants 2 through 4 years of age following administration of the Pfizer-BioNTech COVID-19 Vaccine and the Pfizer-BioNTech COVID-19 Vaccine, Bivalent included pain at the injection site, fatigue, diarrhea, injection site redness, fever, headache, injection site swelling, chills, vomiting, muscle pain, joint pain, and lymphadenopathy (see Full EUA Prescribing Information).
Adverse reactions in participants 5 through 11 years of age following administration of the Pfizer-BioNTech COVID-19 Vaccine, Bivalent included injection site pain, fatigue, headache, muscle pain, joint pain, chills, vomiting, diarrhea, injection site redness, fever, injection site swelling, and lymphadenopathy (see Full EUA Prescribing Information).
Adverse reactions in participants 12 years of age and older following administration the Pfizer-BioNTech COVID-19 Vaccine, Bivalent included injection site pain, fatigue, headache, muscle pain, chills, joint pain, diarrhea, fever, injection site swelling, injection site redness, vomiting, and lymphadenopathy (see Full EUA Prescribing Information).
Adverse reactions in participants greater than 55 years of age following administration of the bivalent vaccine (Original and Omicron BA.1) included pain at the injection site, fatigue, headache, muscle pain, chills, joint pain, injection site redness, injection site swelling, fever, lymphadenopathy, nausea, and malaise.
Adverse Reactions in Post Authorization Experience
Severe allergic reactions, including anaphylaxis, and other hypersensitivity reactions (e.g., rash, pruritus, urticaria, angioedema), diarrhea, vomiting, pain in extremity (arm), syncope, and dizziness have been reported following administration of the Pfizer-BioNTech COVID-19 Vaccine and the Pfizer-BioNTech COVID-19 Vaccine, Bivalent.
Myocarditis and pericarditis have been reported following administration of the Pfizer-BioNTech COVID-19 Vaccine and the Pfizer-BioNTech COVID-19 Vaccine, Bivalent.
Additional adverse reactions, some of which may be serious, may become apparent with more widespread use of the Pfizer-BioNTech COVID-19 Vaccine or Pfizer-BioNTech COVID-19 Vaccine, Bivalent.
Use with Other Vaccines
There is no information on the co-administration of the Pfizer-BioNTech COVID-19 Vaccine or the Pfizer-BioNTech COVID-19 Vaccine, Bivalent with other vaccines.
INFORMATION TO PROVIDE TO VACCINE RECIPIENTS/CAREGIVERS
As the vaccination provider, you must communicate to the caregiver, information consistent with the "Fact Sheet for Recipients and Caregivers" (and provide a copy or direct the caregiver to the website www.cvdvaccine.com to obtain the Fact Sheet for Recipients and Caregivers) prior to the individual receiving each dose of Pfizer-BioNTech COVID-19 Vaccine or Pfizer-BioNTech COVID-19 Vaccine, Bivalent, including:
- •
- FDA has authorized the emergency use of the Pfizer-BioNTech COVID-19 Vaccine and the Pfizer-BioNTech COVID-19 Vaccine, Bivalent, which are not FDA-approved vaccines.
- •
- There is an option to accept or refuse Pfizer-BioNTech COVID-19 Vaccine or Pfizer-BioNTech COVID-19 Vaccine, Bivalent.
- •
- The significant known and potential risks and benefits of Pfizer-BioNTech COVID-19 Vaccine and Pfizer-BioNTech COVID-19 Vaccine, Bivalent, and the extent to which such risks and benefits are unknown.
- •
- Information about available alternative vaccines and the risks and benefits of those alternatives.
For information on clinical trials that are testing the use of the Pfizer-BioNTech COVID-19 Vaccine or the Pfizer-BioNTech COVID-19 Vaccine, Bivalent to prevent COVID-19, please see www.clinicaltrials.gov.
Provide a vaccination card to the caregiver with the date when the recipient needs to return for the next dose of the primary series.
Provide the v-safe information sheet to caregivers and encourage caregivers to participate in v-safe on behalf of the recipient. V-safe is a new voluntary smartphone-based tool that uses text messaging and web surveys to check in with people who have been vaccinated to identify potential side effects after COVID-19 vaccination. V-safe asks questions that help CDC monitor the safety of COVID-19 vaccines. V-safe also provides dose reminders if needed and live telephone follow-up by CDC if participants report a significant health impact following COVID-19 vaccination. For more information, visit: www.cdc.gov/vsafe.
MANDATORY REQUIREMENTS FOR PFIZER-BIONTECH COVID-19 VACCINE AND PFIZER-BIONTECH COVID-19 VACCINE, BIVALENT ADMINISTRATION UNDER EMERGENCY USE AUTHORIZATION
In order to mitigate the risks of using this unapproved product under EUA and to optimize the potential benefit of Pfizer-BioNTech COVID-19 Vaccine and Pfizer-BioNTech COVID-19 Vaccine, Bivalent, the following items are required. Use of unapproved Pfizer-BioNTech COVID-19 Vaccine or Pfizer-BioNTech COVID-19 Vaccine, Bivalent for active immunization to prevent COVID-19 under this EUA is limited to the following (all requirements must be met):
- 1.
- Pfizer-BioNTech COVID-19 Vaccine and Pfizer-BioNTech COVID-19 Vaccine, Bivalent are authorized for use in individuals 6 months of age and older.
- 2.
- The vaccination provider must communicate to the individual receiving the Pfizer-BioNTech COVID-19 Vaccine or the Pfizer-BioNTech COVID-19 Vaccine, Bivalent or their caregiver, information consistent with the "Fact Sheet for Recipients and Caregivers" prior to the individual receiving Pfizer-BioNTech COVID-19 Vaccine.
- 3.
- The vaccination provider must include vaccination information in the state/local jurisdiction's Immunization Information System (IIS) or other designated system.
- 4.
- The vaccination provider is responsible for mandatory reporting of the following to the Vaccine Adverse Event Reporting System (VAERS):
- •
- vaccine administration errors whether or not associated with an adverse event,
- •
- serious adverse events* (irrespective of attribution to vaccination),
- •
- cases of myocarditis,
- •
- cases of pericarditis,
- •
- cases of Multisystem Inflammatory Syndrome (MIS) in adults and children, and
- •
- cases of COVID-19 that result in hospitalization or death.
Complete and submit reports to VAERS online at https://vaers.hhs.gov/reportevent.html. For further assistance with reporting to VAERS call 1-800-822-7967. The reports should include the words "Pfizer-BioNTech COVID-19 Vaccine EUA" in the description section of the report.
- 5.
- The vaccination provider is responsible for responding to FDA requests for information about vaccine administration errors, adverse events, cases of myocarditis, cases of pericarditis, cases of MIS in adults and children, and cases of COVID-19 that result in hospitalization or death following administration of Pfizer-BioNTech COVID-19 Vaccine or Pfizer-BioNTech COVID-19 Vaccine, Bivalent to recipients.
* Serious adverse events are defined as:
- •
- Death;
- •
- A life-threatening adverse event;
- •
- Inpatient hospitalization or prolongation of existing hospitalization;
- •
- A persistent or significant incapacity or substantial disruption of the ability to conduct normal life functions;
- •
- A congenital anomaly/birth defect;
- •
- An important medical event that based on appropriate medical judgement may jeopardize the individual and may require medical or surgical intervention to prevent 1 of the outcomes listed above.
OTHER ADVERSE EVENT REPORTING TO VAERS AND PFIZER INC.
Vaccination providers may report to VAERS other adverse events that are not required to be reported using the contact information above.
To the extent feasible, report adverse events to Pfizer Inc. using the contact information below or by providing a copy of the VAERS form to Pfizer Inc.
| Website | Fax number | Telephone number |
|---|---|---|
|
1-866-635-8337 |
1-800-438-1985 |
ADDITIONAL INFORMATION
For general questions, visit the website or call the telephone number provided below.
To access the most recent Pfizer-BioNTech COVID-19 Vaccine or Pfizer-BioNTech COVID-19 Vaccine, Bivalent Fact Sheets, please scan the QR code provided below.
| Global website | Telephone number |
|---|---|
|
1-877-829-2619 |
AVAILABLE ALTERNATIVES
There may be clinical trials or availability under EUA of other COVID-19 vaccines.
FEDERAL COVID-19 VACCINATION PROGRAM
This vaccine is being made available for emergency use exclusively through the CDC COVID-19 Vaccination Program (the Vaccination Program). Healthcare providers must enroll as providers in the Vaccination Program and comply with the provider requirements. Vaccination providers may not charge any fee for the vaccine and may not charge the vaccine recipient any out-of-pocket charge for administration. However, vaccination providers may seek appropriate reimbursement from a program or plan that covers COVID-19 vaccine administration fees for the vaccine recipient (private insurance, Medicare, Medicaid, Health Resources & Services Administration [HRSA] COVID-19 Uninsured Program for non-insured recipients). For information regarding provider requirements and enrollment in the CDC COVID-19 Vaccination Program, see https://www.cdc.gov/vaccines/covid-19/provider-enrollment.html.
Individuals becoming aware of any potential violations of the CDC COVID-19 Vaccination Program requirements are encouraged to report them to the Office of the Inspector General, U.S. Department of Health and Human Services, at 1-800-HHS-TIPS or https://TIPS.HHS.GOV.
AUTHORITY FOR ISSUANCE OF THE EUA
The Secretary of Health and Human Services (HHS) has declared a public health emergency that justifies the emergency use of drugs and biological products during the COVID-19 pandemic. In response, FDA has issued an EUA for the unapproved product, Pfizer-BioNTech COVID-19 Vaccine and Pfizer-BioNTech COVID-19 Vaccine, Bivalent for active immunization to prevent COVID-19.
FDA issued this EUA, based on Pfizer-BioNTech's request and submitted data.
For the authorized uses, although limited scientific information is available, based on the totality of the scientific evidence available to date, it is reasonable to believe that the Pfizer-BioNTech COVID-19 Vaccine and the Pfizer-BioNTech COVID-19 Vaccine, Bivalent may be effective for the prevention of COVID-19 in individuals as specified in the Full EUA Prescribing Information.
This EUA for the Pfizer-BioNTech COVID-19 Vaccine and the Pfizer-BioNTech COVID-19 Vaccine, Bivalent will end when the Secretary of HHS determines that the circumstances justifying the EUA no longer exist or when there is a change in the approval status of the product such that an EUA is no longer needed.
For additional information about Emergency Use Authorization visit FDA at: https://www.fda.gov/emergency-preparedness-and-response/mcm-legal-regulatory-and-policy-framework/emergency-use-authorization.
The Countermeasures Injury Compensation Program
The Countermeasures Injury Compensation Program (CICP) is a federal program that has been created to help pay for related costs of medical care and other specific expenses to compensate people injured after use of certain medical countermeasures. Medical countermeasures are specific vaccines, medications, devices, or other items used to prevent, diagnose, or treat the public during a public health emergency or a security threat. For more information about CICP regarding the Pfizer-BioNTech COVID-19 Vaccine used to prevent COVID-19, visit www.hrsa.gov/cicp, email cicp@hrsa.gov, or call: 1-855-266-2427.

Manufactured for
BioNTech Manufacturing GmbH
An der Goldgrube 12
55131 Mainz, Germany

Manufactured by
Pfizer Inc., New York, NY 10001
LAB-1516-5.0
Revised: 14 March 2023
END SHORT VERSION FACT SHEET
Long Version (Full EUA Prescribing Information) Begins On Next Page
- 1
- The Pfizer-BioNTech COVID-19 Vaccine is a monovalent vaccine that encodes the spike protein of only the SARS-CoV-2 Wuhan-Hu-1 strain (Original strain).
- 2
- The Pfizer-BioNTech COVID-19 Vaccine, Bivalent encodes the spike protein of the Original SARS-CoV-2 and Omicron BA.4/BA.5 SARS-CoV-2.
- 3
- The Pfizer-BioNTech COVID-19 Vaccine is no longer authorized for use as the third dose of the primary series in individuals 6 months through 4 years of age.
- 4
- Please refer to the Summary of Instructions for COVID-19 Vaccination Providers below for instructions for individuals who will turn from 4 years to 5 years of age between any doses in the primary series.
- 5
- Notwithstanding the age limitations for use of the different vaccines described above, individuals turning from 4 to 5 years of age between any doses in the primary series who previously received Pfizer-BioNTech COVID-19 Vaccine (supplied in multiple dose vials with maroon caps and labels with maroon borders) for Dose 1 and Pfizer-BioNTech COVID-19 Vaccine (supplied in multiple dose vials with orange caps and labels with orange borders) for Dose 2, should receive Pfizer-BioNTech
|
FULL EMERGENCY USE AUTHORIZATION (EUA) PRESCRIBING INFORMATION | |
|
PFIZER-BIONTECH COVID-19 VACCINE AND PFIZER-BIONTECH COVID-19 VACCINE, BIVALENT | |
|
FULL EMERGENCY USE AUTHORIZATION PRESCRIBING INFORMATION: CONTENTS*
|
* Sections or subsections omitted from the full emergency use authorization prescribing information are not listed. |
1 AUTHORIZED USE
Pfizer-BioNTech COVID-19 Vaccine and Pfizer-BioNTech COVID-19 Vaccine, Bivalent (Original and Omicron BA.4/BA.5) are authorized for use under an Emergency Use Authorization (EUA) for active immunization to prevent coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in individuals 6 months of age and older.
This EUA Prescribing Information pertains only to Pfizer-BioNTech COVID-19 Vaccine and Pfizer-BioNTech COVID-19 Vaccine, Bivalent (Original and Omicron BA.4/BA.5), hereafter referred to as Pfizer-BioNTech COVID-19 Vaccine, Bivalent, supplied in multiple dose vials with maroon caps and labels with maroon borders, which are authorized for use in individuals 6 months through 4 years of age.
2 DOSAGE AND ADMINISTRATION
For intramuscular injection only.
The storage, preparation, and administration information in this Prescribing Information apply to the Pfizer-BioNTech COVID-19 Vaccine and the Pfizer-BioNTech COVID-19 Vaccine, Bivalent which are supplied in multiple dose vials with maroon caps and labels with maroon borders.
| Age Range | Dilution Information | Doses Per Vial After Dilution | Dose Volume |
|---|---|---|---|
|
|||
|
6 months through 4 years* |
Dilute with 2.2 mL sterile 0.9% Sodium Chloride Injection, USP prior to use |
10 |
0.2 mL |
2.1 Preparation for Administration
Each vial MUST BE DILUTED before administering the vaccine.
Prior to Dilution
- •
- The Pfizer-BioNTech COVID-19 Vaccine and the Pfizer-BioNTech COVID-19 Vaccine, Bivalent multiple dose vials with maroon caps and labels with maroon borders contain a volume of 0.4 mL, and are supplied as frozen suspensions that do not contain preservative.
- •
- Each vial must be thawed before dilution.
- o
- Vials may be thawed in the refrigerator [2ºC to 8ºC (35ºF to 46ºF)] or at room temperature [up to 25ºC (77ºF)].
- o
- Refer to thawing instructions in the panels below.
Dilution
- •
- Dilute the vial contents using 2.2 mL of sterile 0.9% Sodium Chloride Injection, USP (not provided) to form the Pfizer-BioNTech COVID-19 Vaccine or the Pfizer-BioNTech COVID-19 Vaccine, Bivalent.
- •
- ONLY use sterile 0.9% Sodium Chloride Injection, USP as the diluent. This diluent is not packaged with the vaccine and must be sourced separately. Do not use bacteriostatic 0.9% Sodium Chloride Injection or any other diluent. Do not add more than 2.2 mL of diluent.
- •
- After dilution, 1 vial contains 10 doses of 0.2 mL.
2.2 Administration Information
Visually inspect each dose in the dosing syringe prior to administration. The vaccine will be a white to off-white suspension. During the visual inspection,
- •
- verify the final dosing volume of 0.2 mL.
- •
- confirm there are no particulates and that no discoloration is observed.
- •
- do not administer if vaccine is discolored or contains particulate matter.
Administer the Pfizer-BioNTech COVID-19 Vaccine or Pfizer-BioNTech COVID-19 Vaccine, Bivalent intramuscularly.
After dilution, vials of Pfizer-BioNTech COVID-19 Vaccine or Pfizer-BioNTech COVID-19 Vaccine, Bivalent with maroon caps and labels with maroon borders contain 10 doses of 0.2 mL of vaccine. Low dead-volume syringes and/or needles can be used to extract 10 doses from a single vial. If standard syringes and needles are used, there may not be sufficient volume to extract 10 doses from a single vial. Irrespective of the type of syringe and needle:
- •
- Each dose must contain 0.2 mL of vaccine.
- •
- If the amount of vaccine remaining in the vial cannot provide a full dose of 0.2 mL, discard the vial and content.
- •
- Do not pool excess vaccine from multiple vials.
2.3 Vaccination Schedule
The Pfizer-BioNTech COVID-19 Vaccine and the Pfizer-BioNTech COVID-19 Vaccine, Bivalent for individuals 6 months through 4 years of age are supplied in multiple dose vials with maroon caps and labels with maroon borders and after dilution are administered intramuscularly. Each dose is 0.2 mL.
Primary Series
- Dose 1: Pfizer-BioNTech COVID-19 Vaccine
- Dose 2: Pfizer-BioNTech COVID-19 Vaccine
- Dose 3: Pfizer-BioNTech COVID-19 Vaccine, Bivalent
Dose 1 and Dose 2 (Pfizer-BioNTech COVID-19 Vaccine) are administered 3 weeks apart. Dose 3 (Pfizer-BioNTech COVID-19 Vaccine, Bivalent) is administered at least 8 weeks after Dose 2.
Individuals who will turn from 4 years to 5 years of age between any doses in the primary series may receive either:
- •
- a 3-dose primary series comprised of Pfizer-BioNTech COVID-19 Vaccine (supplied in multiple dose vials with maroon caps and labels with maroon borders) for Doses 1 and 2 and Pfizer-BioNTech COVID-19 Vaccine, Bivalent (supplied in multiple dose vials with maroon caps and labels with maroon borders) for Dose 3, or
- •
- a 2-dose primary series with the Pfizer-BioNTech COVID-19 Vaccine authorized for use for individuals 5 through 11 years of age (supplied in multiple dose vials with orange caps and labels with orange borders).
Booster Dose
The Pfizer-BioNTech COVID-19 Vaccine, Bivalent is authorized for use in individuals 6 months through 4 years of age to provide a single booster dose (0.2 mL) at least 2 months after completion of primary vaccination with 3 doses of the Pfizer-BioNTech COVID-19 Vaccine.
3 DOSAGE FORMS AND STRENGTHS
Pfizer-BioNTech COVID-19 Vaccine and Pfizer-BioNTech COVID-19 Vaccine, Bivalent are suspensions for injection.
After preparation, each dose of the Pfizer-BioNTech COVID-19 Vaccine and the Pfizer-BioNTech COVID-19 Vaccine, Bivalent supplied in multiple dose vials with maroon caps and labels with maroon borders is 0.2 mL for individuals 6 months through 4 years of age [see Dosage and Administration (2.1)].
4 CONTRAINDICATIONS
Do not administer Pfizer-BioNTech COVID-19 Vaccine or Pfizer-BioNTech COVID-19 Vaccine, Bivalent to individuals with known history of a severe allergic reaction (e.g., anaphylaxis) to any component of the Pfizer-BioNTech COVID-19 Vaccine [see Description (13)].
5 WARNINGS AND PRECAUTIONS
5.1 Management of Acute Allergic Reactions
Appropriate medical treatment used to manage immediate allergic reactions must be immediately available in the event an acute anaphylactic reaction occurs following administration of Pfizer-BioNTech COVID-19 Vaccine or Pfizer-BioNTech COVID-19 Vaccine, Bivalent.
Monitor Pfizer-BioNTech COVID-19 Vaccine and Pfizer-BioNTech COVID-19 Vaccine, Bivalent recipients for the occurrence of immediate adverse reactions according to the Centers for Disease Control and Prevention (CDC) guidelines (https://www.cdc.gov/vaccines/covid-19/clinical-considerations/managing-anaphylaxis.html).
5.2 Myocarditis and Pericarditis
Postmarketing safety data with Pfizer-BioNTech COVID-19 Vaccine are relevant to Pfizer-BioNTech COVID-19 Vaccine, Bivalent because these vaccines are manufactured using the same process.
Postmarketing data with authorized or approved monovalent mRNA COVID-19 vaccines demonstrate increased risks of myocarditis and pericarditis, particularly within the first week following receipt of the second dose of a 2-dose primary series or the first booster dose (third dose), with most booster doses likely administered at least 5 months after completing primary vaccination. For the Pfizer-BioNTech COVID-19 Vaccine, the observed risk is highest in males 12 through 17 years of age. Although some cases required intensive care support, available data from short-term follow-up suggest that most individuals have had resolution of symptoms with conservative management. Information is not yet available about potential long-term sequelae. The CDC has published considerations related to myocarditis and pericarditis after vaccination, including for vaccination of individuals with a history of myocarditis or pericarditis (https://www.cdc.gov/vaccines/covid-19/clinical-considerations/myocarditis.html).
5.3 Syncope
Syncope (fainting) may occur in association with administration of injectable vaccines, in particular in adolescents. Procedures should be in place to avoid injury from fainting.
6 OVERALL SAFETY SUMMARY
It is MANDATORY for vaccination providers to report to the Vaccine Adverse Event Reporting System (VAERS) all vaccine administration errors, all serious adverse events, cases of myocarditis, cases of pericarditis, cases of Multisystem Inflammatory Syndrome (MIS) in adults and children, and hospitalized or fatal cases of COVID-19 following vaccination with the Pfizer-BioNTech COVID-19 Vaccine or the Pfizer-BioNTech COVID-19 Vaccine, Bivalent. To the extent feasible, provide a copy of the VAERS form to Pfizer Inc. Please see the REQUIREMENTS AND INSTRUCTIONS FOR REPORTING ADVERSE EVENTS AND VACCINE ADMINISTRATION ERRORS section for details on reporting to VAERS and Pfizer Inc.
The safety of Pfizer-BioNTech COVID-19 Vaccine for the first and second dose of the primary series in individuals 6 months through 4 years of age is based on:
- •
- safety data from a clinical study which evaluated primary vaccination with Pfizer-BioNTech COVID-19 Vaccine in individuals 6 months through 4 years of age,
- •
- safety data from clinical studies which evaluated primary vaccination with Pfizer-BioNTech COVID-19 Vaccine in individuals 5 years of age and older, and
- •
- postmarketing safety data with the Pfizer-BioNTech COVID-19 Vaccine.
The safety of the Pfizer-BioNTech COVID-19 Vaccine, Bivalent for the third dose of the primary series or booster dose in individuals 6 months through 4 years of age is based on:
- •
- safety data from clinical studies which evaluated a booster dose of Pfizer-BioNTech COVID-19 Vaccine, Bivalent (Original and Omicron BA.4/BA.5) in individuals 6 months of age and older,
- •
- safety data from a clinical study which evaluated a booster dose with Pfizer-BioNTech's bivalent COVID-19 vaccine (Original and Omicron BA.1), not authorized or approved, hereafter referred to as bivalent vaccine (Original and Omicron BA.1) in individuals greater than 55 years of age,
- o
- The safety data accrued with the bivalent vaccine (Original and Omicron BA.1) and with the Pfizer-BioNTech COVID-19 Vaccine are relevant to the Pfizer-BioNTech COVID-19 Vaccine, Bivalent because these vaccines are manufactured using the same process.
- •
- safety data from clinical studies which evaluated primary vaccination with Pfizer-BioNTech COVID-19 Vaccine in individuals 6 months of age and older and
- •
- safety data from clinical studies which evaluated booster vaccination with Pfizer-BioNTech COVID-19 Vaccine (previously, but no longer, authorized) in individuals 5 years of age and older, and
- •
- postmarketing safety data with the Pfizer-BioNTech COVID-19 Vaccine and the Pfizer-BioNTech COVID-19 Vaccine, Bivalent.
Pfizer-BioNTech COVID-19 Vaccine
In a clinical study C4591007 (Study 3) in participants 6 through 23 months of age who received Pfizer-BioNTech COVID-19 Vaccine containing 3 mcg of a nucleoside-modified messenger RNA encoding the spike (S) glycoprotein of SARS-CoV-2 (3 mcg nucleoside-modified messenger RNA (modRNA)), adverse reactions following administration of any dose included irritability (68.4%), decreased appetite (38.6%), tenderness at the injection site (26.4%), injection site redness (17.8%), fever (14.4%), injection site swelling (7.3%), and lymphadenopathy (0.2%).
In a clinical study C4591007 (Study 3) in participants 2 through 4 years of age who received Pfizer-BioNTech COVID-19 Vaccine (3 mcg modRNA), adverse reactions following administration of any dose included pain at the injection site (47.0%), fatigue (44.8%), injection site redness (18.9%), fever (10.5%), headache (8.7%), injection site swelling (8.4%), chills (5.7%), muscle pain (5.0%), joint pain (2.4%), and lymphadenopathy (0.1%).
Pfizer-BioNTech COVID-19 Vaccine, Bivalent (Original and Omicron BA.4/BA.5)
The Pfizer-BioNTech COVID-19 Vaccine, Bivalent (Original and Omicron BA.4/BA.5) is made up of equal parts of modRNA encoding the S-glycoprotein of SARS-CoV-2 Wuhan-Hu1 strain (Original) and modRNA encoding the S-glycoprotein of SARS-CoV-2 Omicron variant lineage BA.4 and BA.5.
In a clinical study C4591048 (Study 6) in participants 6 through 23 months of age who received a booster (fourth dose) of Pfizer-BioNTech COVID-19 Vaccine, Bivalent (3 mcg modRNA), adverse reactions following administration of the vaccine included irritability (18.2%), drowsiness (9.1%), injection site redness (8.3%), decreased appetite (4.5%), fatigue (4.2%), fever (4.2%), pain at the injection site (4.2%), and injection site swelling (4.2%).
In a clinical study C4591048 (Study 6) in participants 2 through 4 years of age who received a booster (fourth dose) of Pfizer-BioNTech COVID-19 Vaccine, Bivalent (3 mcg modRNA), adverse reactions following administration of the vaccine included fatigue (30.6%), pain at the injection site (27.8%), injection site redness (8.3%), diarrhea (5.6%), injection site swelling (2.8%), vomiting (2.8%) headache (2.8%), joint pain (2.8%), and chills (2.8%).
In a clinical study C4591048 (Study 6) in participants 5 through 11 years of age who received a booster (fourth dose) of Pfizer-BioNTech COVID-19 Vaccine, Bivalent (10 mcg modRNA), adverse reactions following administration of the vaccine included pain at the injection site (64.0%), fatigue (40.5%), headache (25.2%), muscle pain (13.5%), joint pain (9.0%), chills (9.0%), injection site redness (7.2%), fever (4.5%), injection site swelling (4.5%), vomiting (3.6%), diarrhea (3.6%), and lymphadenopathy (0.9%).
In a clinical study C4591044 (Study 5) in participants 12 years of age and older who received a booster (fourth dose) of Pfizer-BioNTech COVID-19 Vaccine, Bivalent (30 mcg modRNA), adverse reactions following administration of the vaccine included pain at the injection site (68.5%), fatigue (56.4%), headache (41.4%), muscle pain (25.8%), chills (16.9%), joint pain (13.4%), diarrhea (9.6%), fever (7.3%), injection site swelling (5.4%), injection site redness (4.5%), vomiting (1.9%), and lymphadenopathy (0.3%).
Bivalent Vaccine (Original and Omicron BA.1)
The clinical study that evaluated a booster dose of the bivalent vaccine (Original and Omicron BA.1) included participants greater than 55 years of age. The bivalent vaccine (Original and Omicron BA.1) contained 15 mcg of modRNA encoding the S-glycoprotein of SARS-CoV-2 Wuhan-Hu-1 strain (Original) and 15 mcg of modRNA encoding the S-glycoprotein of SARS-CoV-2 Omicron variant lineage BA.1, for a total of 30 mcg modRNA per dose. Adverse reactions following administration of the bivalent vaccine (Original and Omicron BA.1) as a second booster dose included pain at the injection site (58.1%), fatigue (49.2%), headache (33.6%), muscle pain (22.3%), chills (13.0%), joint pain (11.3%), injection site redness (7.0%), injection site swelling (6.6%), fever (5.0%), lymphadenopathy (0.3%), nausea (0.3%), and malaise (0.3%).
Post Authorization Experience
Severe allergic reactions, including anaphylaxis, have been reported following administration of the Pfizer-BioNTech COVID-19 Vaccine and the Pfizer-BioNTech COVID-19 Vaccine, Bivalent.
Myocarditis and pericarditis have been reported following administration of the Pfizer-BioNTech COVID-19 Vaccine and the Pfizer-BioNTech COVID-19 Vaccine, Bivalent.
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Pfizer-BioNTech COVID-19 Vaccine
The safety of the primary series Pfizer-BioNTech COVID-19 Vaccine was evaluated in participants 6 months of age and older in 3 clinical studies conducted in the United States, Europe, Turkey, South Africa, and South America.
Study BNT162-01 (Study 1) was a Phase 1/2, 2-part, dose-escalation trial that enrolled 60 participants, 18 through 55 years of age. Study C4591001 (Study 2) is a Phase 1/2/3, multicenter, multinational, randomized, saline placebo-controlled, observer-blind, dose-finding, vaccine candidate-selection (Phase 1) and efficacy (Phase 2/3) study that has enrolled approximately 46,000 participants, 12 years of age or older. Of these, approximately 43,448 participants [21,720 Pfizer-BioNTech COVID-19 Vaccine (30 mcg modRNA); 21,728 placebo] in Phase 2/3 are 16 years of age or older (including 138 and 145 participants 16 and 17 years of age in the vaccine and placebo groups, respectively) and 2,260 participants are 12 through 15 years of age (1,131 and 1,129 in the vaccine and placebo groups, respectively). Study C4591007 (Study 3) is a Phase 1/2/3 multicenter, randomized, dose-finding, open-label (Phase 1) and multinational, saline placebo-controlled, observer-blind, immunogenicity and efficacy (Phase 2/3) study that has enrolled 4,695 participants 5 through 11 years of age, of whom 3,109 participants received Pfizer-BioNTech COVID-19 Vaccine (10 mcg modRNA) and 1,538 participants received placebo in Phase 2/3. Study 3 also enrolled 1,776 participants 6 through 23 months of age, of whom 1,178 participants were in the Pfizer-BioNTech COVID-19 Vaccine (3 mcg modRNA) group and 598 participants in the placebo group; and also enrolled 2,750 participants 2 through 4 years of age, of whom 1,835 participants were in the Pfizer-BioNTech COVID-19 Vaccine group and 915 participants in the placebo group in Phase 2/3.
In Study 2 and Study 3, all participants 6 months through 4 years of age, 5 through 11 years of age, 12 through 15 years of age, and a subset of participants 16 years of age and older, were monitored for solicited local and systemic reactions and use of antipyretic medication after each vaccination in an electronic diary. Participants are being monitored for unsolicited adverse events, including serious adverse events, throughout the study [from Dose 1 through 1 month after the last vaccination (all unsolicited adverse events) or 6 months (serious adverse events) after the last vaccination]. Tables 1 through 4 present the frequency and severity of solicited local and systemic reactions, within 7 days following each dose of Pfizer-BioNTech COVID-19 Vaccine and placebo in participants 6 months through 4 years of age.
Participants 6 Through 23 Months of Age (3-Dose Primary Series)
In an analysis of Study 3 (Phase 2/3), based on data in the blinded placebo-controlled follow-up period up to the cutoff date of April 29, 2022, 570 participants 6 through 23 months of age who received a 3-dose primary series [386 Pfizer-BioNTech COVID-19 Vaccine (3 mcg modRNA); 184 placebo] have been followed for a median of 1.3 months after the third dose.
Demographic characteristics in Study 3 were generally similar with regard to age, gender, race, and ethnicity among participants 6 through 23 months of age who received Pfizer-BioNTech COVID-19 Vaccine and those who received placebo. Among the 1,178 participants 6 through 23 months of age who received at least 1 dose of the Pfizer-BioNTech COVID-19 Vaccine, 50.0% were male and 50.0% were female, 78.3% were White, 9.9% were multi-racial, 13.7% were Hispanic/Latino, 7.7% were Asian, 3.6% were Black or African American, and 0.3% were American Indian/Alaska Native.
Solicited Local and Systemic Adverse Reactions
The mean duration of tenderness at the injection site after Dose 3 was 1.5 days (range 1 to 9 days), for redness 1.5 days (range 1 to 5 days), and for swelling 1.8 days (range 1 to 3 days) for participants 6 through 23 months of age in the Pfizer-BioNTech COVID-19 Vaccine group in the blinded placebo-controlled follow-up period (cutoff date of April 29, 2022).
| Note: Reactions were collected in an electronic diary (e-diary) from Day 1 to Day 7 after vaccination. | ||||||
|
||||||
|
Pfizer-BioNTech COVID-19 Vaccine†
|
Pfizer-BioNTech COVID-19 Vaccine†
|
Pfizer-BioNTech COVID-19 Vaccine†
| ||||
|
Redness¶ |
||||||
|
Any |
124 (10.6) |
44 (7.4) |
107 (9.3) |
39 (6.6) |
26 (7.1) |
9 (5.3) |
|
Mild |
114 (9.7) |
41 (6.9) |
97 (8.5) |
36 (6.1) |
17 (4.7) |
8 (4.7) |
|
Moderate |
10 (0.9) |
3 (0.5) |
10 (0.9) |
3 (0.5) |
8 (2.2) |
1 (0.6) |
|
Severe |
0 |
0 |
0 |
0 |
1 (0.3) |
0 |
|
Swelling¶ |
||||||
|
Any |
46 (3.9) |
15 (2.5) |
45 (3.9) |
9 (1.5) |
10 (2.7) |
3 (1.8) |
|
Mild |
40 (3.4) |
13 (2.2) |
39 (3.4) |
8 (1.4) |
7 (1.9) |
3 (1.8) |
|
Moderate |
6 (0.5) |
2 (0.3) |
6 (0.5) |
1 (0.2) |
3 (0.8) |
0 |
|
Severe |
0 |
0 |
0 |
0 |
0 |
0 |
|
Tenderness at the injection site# |
||||||
|
Any |
192 (16.6) |
66 (11.2) |
171 (15.0) |
50 (8.5) |
58 (16.0) |
20 (11.8) |
|
Mild |
181 (15.6) |
61 (10.3) |
154 (13.5) |
42 (7.1) |
51 (14.1) |
17 (10.0) |
|
Moderate |
11 (0.9) |
5 (0.8) |
16 (1.4) |
8 (1.4) |
7 (1.9) |
3 (1.8) |
|
Severe |
0 |
0 |
1 (0.1) |
0 |
0 |
0 |
| Note: Events and use of antipyretic or pain medication were collected in an electronic diary (e-diary) from Day 1 to Day 7 after each dose. | ||||||
|
||||||
|
Pfizer-BioNTech COVID-19 Vaccine†
|
Pfizer-BioNTech COVID-19 Vaccine†
|
Pfizer-BioNTech COVID-19 Vaccine†
| ||||
|
Fever |
||||||
|
≥38.0℃ |
85 (7.2) |
43 (7.2) |
85 (7.4) |
36 (6.1) |
25 (6.8) |
10 (5.9) |
|
≥38.0℃ to 38.4℃ |
42 (3.6) |
22 (3.7) |
41 (3.6) |
18 (3.0) |
14 (3.8) |
7 (4.1) |
|
>38.4℃ to 38.9℃ |
23 (2.0) |
14 (2.4) |
20 (1.7) |
11 (1.9) |
5 (1.4) |
2 (1.2) |
|
>38.9℃ to 40.0℃ |
19 (1.6) |
6 (1.0) |
23 (2.0) |
7 (1.2) |
5 (1.4) |
1 (0.6) |
|
>40.0℃ |
1 (0.1) |
1 (0.2) |
1 (0.1) |
0 |
1 (0.3) |
0 |
|
Decreased appetite¶ |
||||||
|
Any |
257 (22.2) |
125 (21.2) |
252 (22.2) |
106 (18.0) |
73 (20.2) |
23 (13.5) |
|
Mild |
138 (11.9) |
73 (12.4) |
157 (13.8) |
63 (10.7) |
42 (11.6) |
13 (7.6) |
|
Moderate |
116 (10.0) |
51 (8.6) |
91 (8.0) |
42 (7.1) |
27 (7.5) |
10 (5.9) |
|
Severe |
3 (0.3) |
1 (0.2) |
4 (0.4) |
1 (0.2) |
4 (1.1) |
0 |
|
Drowsiness# |
||||||
|
Any |
313 (27.0) |
173 (29.3) |
271 (23.8) |
125 (21.2) |
72 (19.9) |
22 (12.9) |
|
Mild |
251 (21.7) |
130 (22.0) |
201 (17.7) |
98 (16.6) |
50 (13.8) |
15 (8.8) |
|
Moderate |
60 (5.2) |
41 (6.9) |
66 (5.8) |
26 (4.4) |
21 (5.8) |
6 (3.5) |
|
Severe |
2 (0.2) |
2 (0.3) |
4 (0.4) |
1 (0.2) |
1 (0.3) |
1 (0.6) |
|
IrritabilityÞ |
||||||
|
Any |
593 (51.2) |
279 (47.2) |
539 (47.4) |
240 (40.7) |
158 (43.6) |
64 (37.6) |
|
Mild |
245 (21.1) |
106 (17.9) |
213 (18.7) |
89 (15.1) |
56 (15.5) |
27 (15.9) |
|
Moderate |
341 (29.4) |
173 (29.3) |
319 (28.1) |
146 (24.7) |
101 (27.9) |
37 (21.8) |
|
Severe |
7 (0.6) |
0 |
7 (0.6) |
5 (0.8) |
1 (0.3) |
0 |
|
Use of antipyretic or pain medicationß |
281 (24.0) |
117 (19.7) |
243 (21.2) |
111 (18.8) |
70 (19.2) |
28 (16.5) |
Unsolicited Adverse Events
In the following analyses of Study 3 in participants 6 through 23 months of age (386 of whom received Pfizer-BioNTech COVID-19 Vaccine and 184 of whom received placebo), 83.7% of participants had at least 30 days of follow-up after Dose 3.
Serious Adverse Events
Serious adverse events from Dose 1 through 1 month after Dose 3, with an overall median of 1.3 months follow-up after Dose 3 were reported by 1.4% of Pfizer-BioNTech COVID-19 Vaccine recipients and by 2.3% of placebo recipients. No serious adverse events were reported that were considered related to vaccination.
Non-Serious Adverse Events
Non-serious adverse events from Dose 1 through up to 1 month after Dose 3, in ongoing follow-up were reported by 29.1% of Pfizer-BioNTech COVID-19 Vaccine recipients and by 26.3% of placebo recipients.
From Dose 1 through 30 days after Dose 3, lymphadenopathy was reported in 2 (0.2%) participants in the Pfizer-BioNTech COVID-19 Vaccine group vs. 0 (0%) in the placebo group. There were no other notable patterns between treatment groups for specific categories of non-serious adverse events that would suggest a causal relationship to Pfizer-BioNTech COVID-19 Vaccine.
Participants 2 Through 4 Years of Age (3-Dose Primary Series)
In an analysis of Study 3 (Phase 2/3), based on data in the blinded placebo-controlled follow-up period up to the cutoff date of April 29, 2022, 886 participants 2 through 4 years of age who received a 3-dose primary series [606 Pfizer-BioNTech COVID-19 Vaccine (3 mcg modRNA); 280 placebo] have been followed a median of 1.4 months after the third dose.
Demographic characteristics in Study 3 were generally similar with regard to age, gender, race, and ethnicity among participants 2 through 4 years of age who received Pfizer-BioNTech COVID-19 Vaccine and those who received placebo. Among the 1,835 participants 2 through 4 years of age who received at least 1 dose of the Pfizer-BioNTech COVID-19 Vaccine, 49.1% were male and 50.9% were female, 80.1% were White, 14.4% were Hispanic/Latino, 7.1% were multi-racial, 6.9% were Asian, 5.1% were Black or African American, and 0.2% were American Indian/Alaska Native.
Solicited Local and Systemic Adverse Reactions
The mean duration of pain at the injection site after Dose 3 was 1.7 days (range 1 to 14 days), for redness 1.5 days (range 1 to 3 days), and for swelling 1.8 days (range 1 to 4 days) for participants 2 through 4 years of age in the Pfizer-BioNTech COVID-19 Vaccine group in the blinded placebo-controlled follow-up period (cutoff date of April 29, 2022).
| Note: Reactions were collected in an electronic diary (e-diary) from Day 1 to Day 7 after vaccination. | ||||||
|
||||||
|
Pfizer-BioNTech COVID-19 Vaccine†
|
Pfizer-BioNTech COVID-19 Vaccine†
|
Pfizer-BioNTech COVID-19 Vaccine†
| ||||
|
Redness¶ | ||||||
|
Any |
160 (8.8) |
77 (8.5) |
202 (11.4) |
50 (5.7) |
60 (10.9) |
9 (3.4) |
|
Mild |
137 (7.5) |
67 (7.4) |
170 (9.6) |
43 (4.9) |
53 (9.6) |
7 (2.7) |
|
Moderate |
22 (1.2) |
9 (1.0) |
31 (1.7) |
7 (0.8) |
7 (1.3) |
2 (0.8) |
|
Severe |
1 (0.1) |
1 (0.1) |
1 (0.1) |
0 |
0 |
0 |
|
Swelling¶ | ||||||
|
Any |
67 (3.7) |
26 (2.9) |
102 (5.7) |
18 (2.1) |
17 (3.1) |
3 (1.1) |
|
Mild |
59 (3.2) |
21 (2.3) |
81 (4.6) |
16 (1.8) |
16 (2.9) |
3 (1.1) |
|
Moderate |
8 (0.4) |
5 (0.6) |
21 (1.2) |
2 (0.2) |
1 (0.2) |
0 |
|
Severe |
0 |
0 |
0 |
0 |
0 |
0 |
|
Pain at the injection site# | ||||||
|
Any |
559 (30.8) |
186 (20.6) |
550 (31.0) |
178 (20.3) |
146 (26.7) |
35 (13.4) |
|
Mild |
522 (28.8) |
178 (19.7) |
514 (29.0) |
169 (19.3) |
130 (23.8) |
33 (12.6) |
|
Moderate |
37 (2.0) |
7 (0.8) |
36 (2.0) |
8 (0.9) |
16 (2.9) |
2 (0.8) |
|
Severe |
0 |
1 (0.1) |
0 |
1 (0.1) |
0 |
0 |
| Note: Events and use of antipyretic or pain medication were collected in an electronic diary (e-diary) from Day 1 to Day 7 after each dose. | ||||||
|
||||||
|
Pfizer-BioNTech COVID-19 Vaccine†
|
Pfizer-BioNTech COVID-19 Vaccine†
|
Pfizer-BioNTech COVID-19 Vaccine†
| ||||
|
Fever | ||||||
|
≥38.0℃ |
95 (5.2) |
48 (5.3) |
88 (4.9) |
46 (5.2) |
28 (5.1) |
11 (4.2) |
|
≥38.0℃ to 38.4℃ |
57 (3.1) |
24 (2.6) |
41 (2.3) |
17 (1.9) |
16 (2.9) |
4 (1.5) |
|
>38.4℃ to 38.9℃ |
24 (1.3) |
16 (1.8) |
26 (1.5) |
21 (2.4) |
8 (1.4) |
4 (1.5) |
|
>38.9℃ to 40.0℃ |
13 (0.7) |
8 (0.9) |
19 (1.1) |
8 (0.9) |
4 (0.7) |
3 (1.1) |
|
>40.0℃ |
1 (0.1) |
0 |
2 (0.1) |
0 |
0 |
0 |
|
Fatigue¶ | ||||||
|
Any |
539 (29.7) |
277 (30.6) |
456 (25.7) |
201 (22.9) |
134 (24.5) |
57 (21.8) |
|
Mild |
335 (18.5) |
176 (19.4) |
267 (15.1) |
120 (13.7) |
87 (15.9) |
35 (13.4) |
|
Moderate |
198 (10.9) |
96 (10.6) |
181 (10.2) |
78 (8.9) |
45 (8.2) |
22 (8.4) |
|
Severe |
6 (0.3) |
5 (0.6) |
8 (0.5) |
3 (0.3) |
2 (0.4) |
0 |
|
Headache¶ | ||||||
|
Any |
81 (4.5) |
44 (4.9) |
81 (4.6) |
36 (4.1) |
27 (4.9) |
11 (4.2) |
|
Mild |
63 (3.5) |
35 (3.9) |
63 (3.6) |
23 (2.6) |
19 (3.5) |
10 (3.8) |
|
Moderate |
18 (1.0) |
8 (0.9) |
18 (1.0) |
12 (1.4) |
8 (1.5) |
1 (0.4) |
|
Severe |
0 |
1 (0.1) |
0 |
1 (0.1) |
0 |
0 |
|
Chills¶ | ||||||
|
Any |
41 (2.3) |
22 (2.4) |
53 (3.0) |
23 (2.6) |
18 (3.3) |
7 (2.7) |
|
Mild |
28 (1.5) |
16 (1.8) |
35 (2.0) |
17 (1.9) |
14 (2.6) |
7 (2.7) |
|
Moderate |
10 (0.6) |
6 (0.7) |
18 (1.0) |
6 (0.7) |
3 (0.5) |
0 |
|
Severe |
3 (0.2) |
0 |
0 |
0 |
1 (0.2) |
0 |
|
Vomiting# | ||||||
|
Any |
54 (3.0) |
24 (2.7) |
61 (3.4) |
29 (3.3) |
9 (1.6) |
10 (3.8) |
|
Mild |
44 (2.4) |
14 (1.5) |
55 (3.1) |
26 (3.0) |
7 (1.3) |
9 (3.4) |
|
Moderate |
10 (0.6) |
10 (1.1) |
6 (0.3) |
3 (0.3) |
2 (0.4) |
1 (0.4) |
|
Severe |
0 |
0 |
0 |
0 |
0 |
0 |
|
DiarrheaÞ | ||||||
|
Any |
139 (7.7) |
72 (8.0) |
118 (6.7) |
64 (7.3) |
28 (5.1) |
13 (5.0) |
|
Mild |
130 (7.2) |
64 (7.1) |
105 (5.9) |
57 (6.5) |
21 (3.8) |
10 (3.8) |
|
Moderate |
9 (0.5) |
8 (0.9) |
12 (0.7) |
7 (0.8) |
7 (1.3) |
3 (1.1) |
|
Severe |
0 |
0 |
1 (0.1) |
0 |
0 |
0 |
|
New or worsened muscle pain¶ | ||||||
|
Any |
43 (2.4) |
15 (1.7) |
46 (2.6) |
21 (2.4) |
11 (2.0) |
4 (1.5) |
|
Mild |
33 (1.8) |
13 (1.4) |
33 (1.9) |
17 (1.9) |
8 (1.5) |
4 (1.5) |
|
Moderate |
9 (0.5) |
2 (0.2) |
13 (0.7) |
4 (0.5) |
3 (0.5) |
0 |
|
Severe |
1 (0.1) |
0 |
0 |
0 |
0 |
0 |
|
New or worsened joint pain¶ | ||||||
|
Any |
14 (0.8) |
18 (2.0) |
24 (1.4) |
9 (1.0) |
7 (1.3) |
2 (0.8) |
|
Mild |
12 (0.7) |
13 (1.4) |
18 (1.0) |
6 (0.7) |
5 (0.9) |
2 (0.8) |
|
Moderate |
2 (0.1) |
5 (0.6) |
6 (0.3) |
3 (0.3) |
1 (0.2) |
0 |
|
Severe |
0 |
0 |
0 |
0 |
1 (0.2) |
0 |
|
Use of antipyretic or pain medicationß |
197 (10.8) |
83 (9.1) |
177 (9.9) |
74 (8.4) |
47 (8.5) |
18 (6.9) |
Unsolicited Adverse Events
In the following analyses of Study 3 in participants 2 through 4 years of age (606 of whom received Pfizer-BioNTech COVID-19 Vaccine and 280 of whom received placebo), 76.6% of participants had at least 30 days of follow-up after Dose 3.
Serious Adverse Events
Serious adverse events from Dose 1 through 1 month after Dose 3, with an overall median of 1.4 months follow-up after Dose 3 were reported by 0.7% of Pfizer-BioNTech COVID-19 Vaccine recipients and by 0.9% of placebo recipients. One serious adverse event of fever (maximum temperature 40.3°C) on Day 3 after Dose 2 in a 4-year-old was considered possibly related to vaccination.
Non-Serious Adverse Events
Non-serious adverse events from Dose 1 through up to 30 days after Dose 3, in ongoing follow-up were reported by 18.5% of Pfizer-BioNTech COVID-19 Vaccine recipients and by 18.5% of placebo recipients.
From Dose 1 through 30 days after Dose 3, lymphadenopathy was reported in 1 (0.1%) participant in the Pfizer-BioNTech COVID-19 Vaccine (3 mcg modRNA) group vs. 0 (0.0%) in the placebo group. There were no other notable patterns between treatment groups for specific categories of non-serious adverse events that would suggest a causal relationship to Pfizer-BioNTech COVID-19 Vaccine.
Participants 5 Through 11 Years of Age (2-Dose Primary Series)
In an analysis of Study 3 (Phase 2/3), based on data up to the cutoff date of September 06, 2021, 2,268 participants [1,518 Pfizer-BioNTech COVID-19 Vaccine (10 mcg modRNA); 750 placebo] were 5 through 11 years of age. Of these, 2,158 (95.1%) [1,444 Pfizer-BioNTech COVID-19 Vaccine (10 mcg modRNA) and 714 placebo] participants have been followed for at least 2 months after the second dose. An analysis of Study 3 Phase 2/3 adverse event data also included another 2,379 participants [1,591 Pfizer-BioNTech COVID-19 Vaccine (10 mcg modRNA) and 788 placebo], of whom 71.2% had a follow-up period for at least 2 weeks after Dose 2 up to the cutoff date of October 8, 2021. The safety evaluation in Study 3 is ongoing.
Demographic characteristics in Study 3 were generally similar with regard to age, gender, race, and ethnicity among participants 5 through 11 years of age who received Pfizer-BioNTech COVID-19 Vaccine (10 mcg modRNA) and those who received placebo. Among the 4,647 participants 5 through 11 years of age who received at least 1 dose of the Pfizer-BioNTech COVID-19 Vaccine (10 mcg modRNA) or placebo, 51.8% were male and 48.2% were female, 77.3% were White, 5.8% were Black or African American, 16.9% were Hispanic/Latino, 8.3% were Asian, and 0.4% were American Indian/Alaska Native.
Unsolicited Adverse Events
In the following analyses of Study 3 in participants 5 through 11 years of age (1,518 of whom received Pfizer-BioNTech COVID-19 Vaccine [10 mcg modRNA] and 750 of whom received placebo), 99.5% of participants had at least 30 days of follow-up after Dose 2.
Serious Adverse Events
In 1 group of participants (initial enrollment cohort) with a median of 2.3 months follow-up post Dose 2, no serious adverse events were reported that were considered related to vaccination. In a second group of participants (expansion cohort) with a median of 2.4 weeks follow-up post Dose 2, no serious adverse events were reported that were considered related to vaccination.
Non-Serious Adverse Events
In 1 group of participants (initial enrollment cohort), non-serious adverse events from Dose 1 through up to 30 days after Dose 2 up to the cutoff date of September 06, 2021, in ongoing follow-up were reported by 10.9% of Pfizer-BioNTech COVID-19 Vaccine (10 mcg modRNA) recipients and by 9.1% of placebo recipients. In this group of participants, >99% had follow-up 30 days post Dose 2. In a second group of participants (expansion cohort) for which the median follow-up was 2.4 weeks (range 0 – 3.7 weeks), non-serious adverse events from Dose 1 through the cutoff date of October 8, 2021, were reported by 7.1% of Pfizer-BioNTech COVID-19 Vaccine (10 mcg modRNA) recipients and by 6.3% of placebo recipients.
In the initial enrollment cohort, from Dose 1 through 30 days after Dose 2, lymphadenopathy was reported in 13 (0.9%) participants in the Pfizer-BioNTech COVID-19 Vaccine (10 mcg modRNA) group vs. 1 (0.1%) in the placebo group. In the expansion cohort from Dose 1 through the cutoff date, lymphadenopathy was reported in 6 (0.4%) participants in the Pfizer-BioNTech COVID-19 Vaccine (10 mcg modRNA) group vs. 3 (0.4%) in the placebo group. There were no other notable patterns between treatment groups for specific categories of non-serious adverse events that would suggest a causal relationship to Pfizer-BioNTech COVID-19 Vaccine.
Participants 12 Through 15 Years of Age (2-Dose Primary Series)
In an analysis of Study 2, based on data up to the cutoff date of March 13, 2021, 2,260 participants [1,131 Pfizer-BioNTech COVID-19 Vaccine (30 mcg modRNA); 1,129 placebo] were 12 through 15 years of age. Of these, 1,308 (660 Pfizer-BioNTech COVID-19 Vaccine and 648 placebo) participants have been followed for at least 2 months after the second dose. The safety evaluation in Study 2 is ongoing.
Demographic characteristics in Study 2 were generally similar with regard to age, gender, race, and ethnicity among participants who received Pfizer-BioNTech COVID-19 Vaccine and those who received placebo. Overall, among the participants who received the Pfizer-BioNTech COVID-19 Vaccine, 50.1% were male and 49.9% were female, 85.9% were White, 4.6% were Black or African American, 11.7% were Hispanic/Latino, 6.4% were Asian, and 0.4% were American Indian/Alaska Native.
Unsolicited Adverse Events
In the following analyses of Study 2 in participants 12 through 15 years of age (1,131 of whom received Pfizer-BioNTech COVID-19 Vaccine and 1,129 of whom received placebo), 98.3% of study participants had at least 30 days of follow-up after Dose 2.
Serious Adverse Events
Serious adverse events from Dose 1 through up to 30 days after Dose 2 in ongoing follow-up were reported by 0.4% of Pfizer-BioNTech COVID-19 Vaccine recipients and by 0.1% of placebo recipients. There were no notable patterns or numerical imbalances between treatment groups for specific categories of serious adverse events that would suggest a causal relationship to Pfizer-BioNTech COVID-19 Vaccine.
Non-Serious Adverse Events
Non-serious adverse events from Dose 1 through up to 30 days after Dose 2 in ongoing follow-up were reported by 5.8% of Pfizer-BioNTech COVID-19 Vaccine recipients and by 5.8% of placebo recipients.
From Dose 1 through 30 days after Dose 2, reports of lymphadenopathy plausibly related to the study intervention were imbalanced, with notably more cases in the Pfizer-BioNTech COVID-19 Vaccine group (7) vs. the placebo group (1). There were no other notable patterns or numerical imbalances between treatment groups for specific categories of non-serious adverse events that would suggest a causal relationship to Pfizer-BioNTech COVID-19 Vaccine.
Participants 16 Years of Age and Older (2-Dose Primary Series)
At the time of the analysis of Study 2 for the EUA, 37,586 [18,801 Pfizer-BioNTech COVID-19 Vaccine (30 mcg modRNA) and 18,785 placebo] participants 16 years of age or older had been followed for a median of 2 months after the second dose.
The safety evaluation in Study 2 is ongoing. The safety population includes participants 16 years and older enrolled by October 9, 2020, and includes safety data accrued through November 14, 2020.
Demographic characteristics in Study 2 were generally similar with regard to age, gender, race, and ethnicity among participants who received Pfizer-BioNTech COVID-19 Vaccine and those who received placebo. Overall, among the total participants who received either the Pfizer-BioNTech COVID-19 Vaccine or placebo, 50.6% were male and 49.4% were female, 83.1% were White, 9.1% were Black or African American, 28.0% were Hispanic/Latino, 4.3% were Asian, and 0.5% were American Indian/Alaska Native.
Unsolicited Adverse Events
Serious Adverse Events
In Study 2, among participants 16 through 55 years of age who had received at least 1 dose of vaccine or placebo (Pfizer-BioNTech COVID-19 Vaccine = 10,841; placebo = 10,851), serious adverse events from Dose 1 through up to 30 days after Dose 2 in ongoing follow-up were reported by 0.4% of Pfizer-BioNTech COVID-19 Vaccine recipients and by 0.3% of placebo recipients. In a similar analysis, in participants 56 years of age and older (Pfizer-BioNTech COVID-19 Vaccine = 7,960, placebo = 7,934), serious adverse events were reported by 0.8% of Pfizer-BioNTech COVID-19 Vaccine recipients and by 0.6% of placebo recipients who received at least 1 dose of Pfizer-BioNTech COVID-19 Vaccine or placebo, respectively. In these analyses, 91.6% of study participants had at least 30 days of follow-up after Dose 2.
Appendicitis was reported as a serious adverse event for 12 participants, and numerically higher in the vaccine group, 8 vaccine participants and 4 placebo participants. Currently available information is insufficient to determine a causal relationship with the vaccine. There were no other notable patterns or numerical imbalances between treatment groups for specific categories of serious adverse events (including neurologic, neuro-inflammatory, and thrombotic events) that would suggest a causal relationship to Pfizer-BioNTech COVID-19 Vaccine.
Non-Serious Adverse Events
In Study 2 in which 10,841 participants 16 through 55 years of age received Pfizer-BioNTech COVID-19 Vaccine and 10,851 participants received placebo, non-serious adverse events from Dose 1 through up to 30 days after Dose 2 in ongoing follow-up were reported in 29.3% of participants who received Pfizer-BioNTech COVID-19 Vaccine and 13.2% of participants in the placebo group, for participants who received at least 1 dose. Overall, in a similar analysis in which 7,960 participants 56 years of age and older received Pfizer-BioNTech COVID-19 Vaccine, non-serious adverse events within 30 days were reported in 23.8% of participants who received Pfizer-BioNTech COVID-19 Vaccine and 11.7% of participants in the placebo group, for participants who received at least 1 dose. In these analyses, 91.6% of study participants had at least 30 days of follow-up after Dose 2.
The higher frequency of reported unsolicited non-serious adverse events among Pfizer-BioNTech COVID-19 Vaccine recipients compared to placebo recipients was primarily attributed to local and systemic adverse events reported during the first 7 days following vaccination that are consistent with adverse reactions solicited among participants in the reactogenicity subset. From Dose 1 through 30 days after Dose 2, reports of lymphadenopathy were imbalanced with notably more cases in the Pfizer-BioNTech COVID-19 Vaccine group (64) vs. the placebo group (6), which is plausibly related to vaccination. Throughout the safety follow-up period to date, Bell's palsy (facial paralysis) was reported by 4 participants in the Pfizer-BioNTech COVID-19 Vaccine group. Onset of facial paralysis was Day 37 after Dose 1 (participant did not receive Dose 2) and Days 3, 9, and 48 after Dose 2. No cases of Bell's palsy were reported in the placebo group. Currently available information is insufficient to determine a causal relationship with the vaccine. There were no other notable patterns or numerical imbalances between treatment groups for specific categories of non-serious adverse events (including other neurologic or neuro-inflammatory, and thrombotic events) that would suggest a causal relationship to Pfizer-BioNTech COVID-19 Vaccine.
Bivalent Vaccine (Original and Omicron BA.1) Administered as a Second Booster Dose in Individuals Greater than 55 Years of Age
In Study 4, a total of 610 participants greater than 55 years of age previously vaccinated with a 2-dose primary series and 1 booster dose of Pfizer-BioNTech COVID-19 Vaccine went on to receive a second booster dose with either Pfizer-BioNTech COVID-19 Vaccine or the bivalent vaccine (Original and Omicron BA.1).
- The 305 participants greater than 55 years who received a second boo ster dose with Pfizer-BioNTech COVID-19 received it 5.3 to 13.1 months after receiving the first booster dose and had a median follow-up time of 1.8 months up to a data cutoff date of May 16, 2022. Their median age was 66 years (range 56 through 87 years of age), 47.5% were male and 52.5% were female, 87.9% were White, 18.7% were Hispanic/Latino, 4.3% were Asian, and 6.2% were Black or African American.
The 305 participants greater than 55 years who received a second booster dose with the bivalent vaccine (Original and Omicron BA.1) received it 4.7 to 11.5 months after receiving the first booster dose and had a median follow-up time of 1.7 months up to a data cutoff date of May 16, 2022. Their median age was 67 years (range 56 through 85 years of age), 53.1% were male and 46.9% were female, 89.8% were White, 14.8% were Hispanic/Latino, 5.2% were Asian, and 4.3% were Black or African American.
Solicited Local and Systemic Adverse Reactions
Table 5 and Table 6 present the frequency and severity of reported solicited local reactions and systemic reactions, respectively, within 7 days of a second booster dose of Pfizer-BioNTech COVID-19 Vaccine or bivalent vaccine (Original and Omicron BA.1).
In participants who received the bivalent vaccine (Original and Omicron BA.1), the mean duration of injection site pain, redness, and swelling was 2.2 days (range 1 to 12 days), 2.9 days (range 1 to 10 days), and 1.9 days (range 1 to 4 days), respectively.
| Note: Adverse Reactions were collected in the electronic diary (e-diary) from day of vaccination (Day 1) through Day 7 after the study vaccination. | ||
|
||
|
Redness‡ |
||
|
Any (>2 cm) |
19 (6.4) |
21 (7.0) |
|
Mild |
12 (4.0) |
13 (4.3) |
|
Moderate |
6 (2.0) |
8 (2.7) |
|
Severe |
1 (0.3) |
0 |
|
Swelling‡ |
||
|
Any (>2 cm) |
18 (6.0) |
20 (6.6) |
|
Mild |
10 (3.4) |
14 (4.7) |
|
Moderate |
8 (2.7) |
6 (2.0) |
|
Severe |
0 |
0 |
|
Pain at the injection site§ |
||
|
Any |
179 (60.1) |
175 (58.1) |
|
Mild |
154 (51.7) |
159 (52.8) |
|
Moderate |
24 (8.1) |
15 (5.0) |
|
Severe |
1 (0.3) |
1 (0.3) |
| Note: Adverse reactions and use of antipyretic or pain medication were collected in the electronic diary (e-diary) from day of vaccination (Day 1) through Day 7 after the study vaccination. | ||
|
||
|
Fever |
||
|
≥38.0°C |
11 (3.7) |
15 (5.0) |
|
≥38.0°C to 38.4°C |
6 (2.0) |
11 (3.7) |
|
>38.4°C to 38.9°C |
5 (1.7) |
0 |
|
>38.9°C to 40.0°C |
0 |
4 (1.3) |
|
>40.0°C |
0 |
0 |
|
Fatigue‡ |
||
|
Any |
135 (45.3) |
148 (49.2) |
|
Mild |
70 (23.5) |
88 (29.2) |
|
Moderate |
64 (21.5) |
55 (18.3) |
|
Severe |
1 (0.3) |
5 (1.7) |
|
Headache‡ |
||
|
Any |
79 (26.5) |
101 (33.6) |
|
Mild |
47 (15.8) |
71 (23.6) |
|
Moderate |
31 (10.4) |
29 (9.6) |
|
Severe |
1 (0.3) |
1 (0.3) |
|
Chills‡ |
||
|
Any |
49 (16.4) |
39 (13.0) |
|
Mild |
32 (10.7) |
25 (8.3) |
|
Moderate |
17 (5.7) |
14 (4.7) |
|
Severe |
0 |
0 |
|
Vomiting§ |
||
|
Any |
4 (1.3) |
5 (1.7) |
|
Mild |
2 (0.7) |
5 (1.7) |
|
Moderate |
2 (0.7) |
0 |
|
Severe |
0 |
0 |
|
Diarrhea¶ |
||
|
Any |
13 (4.4) |
27 (9.0) |
|
Mild |
10 (3.4) |
18 (6.0) |
|
Moderate |
3 (1.0) |
5 (1.7) |
|
Severe |
0 |
4 (1.3) |
|
New or worsened muscle pain‡ |
||
|
Any |
59 (19.8) |
67 (22.3) |
|
Mild |
35 (11.7) |
40 (13.3) |
|
Moderate |
24 (8.1) |
27 (9.0) |
|
Severe |
0 |
0 |
|
New or worsened joint pain‡ |
||
|
Any |
27 (9.1) |
34 (11.3) |
|
Mild |
16 (5.4) |
23 (7.6) |
|
Moderate |
11 (3.7) |
11 (3.7) |
|
Severe |
0 |
0 |
|
Use of antipyretic or pain medication# |
80 (26.8) |
88 (29.2) |
Unsolicited Adverse Events
Overall, the participants who received a second booster dose with the bivalent vaccine (Original and Omicron BA.1) had a median follow-up time of 1.7 months (range 1.0 to 2.0 months) to the cutoff date (May 16, 2022).
In an analysis of all unsolicited adverse events reported following the second booster dose, through 1 month after the booster dose, those assessed as adverse reactions not already captured by solicited local and systemic reactions were lymphadenopathy (n = 1; 0.3%) for the Pfizer-BioNTech COVID-19 Vaccine and (n = 1; 0.3%) for the bivalent vaccine (Original and Omicron BA.1), nausea (n = 1; 0.3%) for the Pfizer-BioNTech COVID-19 Vaccine and (n = 1; 0.3%) for the bivalent vaccine (Original and Omicron BA.1), and malaise (n = 0) for the Pfizer-BioNTech COVID-19 Vaccine and (n = 1; 0.3%) for the bivalent vaccine (Original and Omicron BA.1).
Serious Adverse Events
Serious adverse events up to 1 month after the second booster dose in ongoing follow-up were reported by no Pfizer-BioNTech COVID-19 Vaccine recipients and by 1 bivalent vaccine (Original and Omicron BA.1) recipient (1 serious adverse event considered unrelated to the vaccine).
Pfizer-BioNTech COVID-19 Vaccine, Bivalent (Original and Omicron BA.4/BA.5)
In Study 6, all participants 6 months through 4 years of age were monitored for solicited local and systemic reactions and use of antipyretic medication after the vaccination in an electronic diary. Participants are being monitored for unsolicited adverse events, including serious adverse events, throughout the study [through 1 month after the booster (fourth dose)]. Tables 7 through 10 present the frequency and severity of solicited local and systemic reactions, within 7 days following a booster (fourth dose) of Pfizer-BioNTech COVID-19 Vaccine, Bivalent in participants 6 through 23 months of age and 2 through 4 years of age who were previously vaccinated with a 3-dose primary series of Pfizer-BioNTech COVID-19 Vaccine.
Participants 6 Through 23 Months of Age Who Received a Booster Dose with Pfizer-BioNTech COVID-19 Vaccine, Bivalent (Original and Omicron BA.4/BA.5)
In a subset of Study 6, 24 participants 6 through 23 months previously vaccinated with a 3-dose primary series of Pfizer-BioNTech COVID-19 Vaccine (3 mcg modRNA) received a booster (fourth dose) of Pfizer-BioNTech COVID-19 Vaccine, Bivalent (Original and Omicron BA.4/BA.5) (3 mcg modRNA).
Participants received a booster dose with Pfizer-BioNTech COVID-19 Vaccine, Bivalent 2.1 to 8.6 months after receiving their third dose with Pfizer-BioNTech COVID-19 and had a median follow-up time of 1.6 months (range 1.5 to 2.3 months) up to a data cutoff date of November 25, 2022. Their median age was 19 months (range 12 through 23 months), 58.3% were female and 41.7% were male, 54.2% were White, 20.8% were Asian, 20.8% were multi-racial, 16.7% were Hispanic/Latino, and 4.2% were Black or African American.
Solicited Local and Systemic Adverse Reactions
Table 7 and Table 8 present the frequency and severity of reported solicited local reactions and systemic reactions, respectively, within 7 days of a booster (fourth dose) of Pfizer-BioNTech COVID-19 Vaccine, Bivalent.
The duration of injection site tenderness, swelling and redness for all events observed was 1 day.
Table 7: Local Adverse Reactions, by Maximum Severity, Within 7 Days After a Booster (Fourth Dose) – Participants 6 through 23 Months of Age – Safety Population
| Reactions were collected in the electronic diary (e-diary) and at unscheduled clinical assessments from Day 1 through Day 7 after the study vaccination. Reactions reported as adverse events in the case report form within 7 days after the study vaccination were also included in the analysis; the severity of these events is based on the grading scale in the adverse event section of the case report form. | |
|
|
|
Pfizer-BioNTech COVID-19 Vaccine, Bivalent (Original and Omicron BA.4/BA.5) 3 mcg modRNA n‡ (%) |
|
|
Redness§ | |
|
Any (≥0.5 cm) |
2 (8.3) |
|
Mild |
2 (8.3) |
|
Swelling§ | |
|
Any (≥0.5 cm) |
1 (4.2) |
|
Mild |
1 (4.2) |
|
Tenderness at the injection site¶ | |
|
Any |
1 (4.3) |
|
Mild |
1 (4.3) |
Table 8: Frequency and Percentages of Participants with Solicited Systemic Reactions, by Maximum Severity, Within 7 Days After a Booster (Fourth Dose) – Participants 6 Through 23 Months of Age – Safety Population
| Note: Events and use of antipyretic or pain medication were collected in the electronic diary (e-diary) and at unscheduled clinical assessments from Day 1 through Day 7 after the study vaccination. Events reported as adverse events in the case report form within 7 days after the study vaccination were also included in the analysis; the severity of these events is based on the grading scale in the adverse event section of the case report form. | |
|
|
|
Pfizer-BioNTech COVID-19 Vaccine, Bivalent (Original and Omicron BA.4/BA.5) 3 mcg modRNA n‡ (%) |
|
|
Fever§ |
|
|
≥38.0℃ |
1 (4.2) |
|
≥38.0℃ to 38.4℃ |
1 (4.2) |
|
Decreased appetite¶ |
|
|
Any |
1 (4.5) |
|
Mild |
1 (4.5) |
|
Drowsiness# |
|
|
Any |
2 (9.1) |
|
Mild |
2 (9.1) |
|
IrritabilityÞ |
|
|
Any |
4 (18.2) |
|
Mild |
3 (13.6) |
|
Moderate |
1 (4.5) |
|
Use of antipyretic or pain medicationß |
2 (8.3) |
Unsolicited Adverse Events
Participants 6 through 23 months of age who received a booster (fourth dose) with the Pfizer-BioNTech COVID-19 Vaccine, Bivalent had a median follow-up time of 1.6 months (range 1.5 to 2.3 months) to the cutoff date (November 25, 2022). In an analysis of all unsolicited adverse events reported following the booster dose through 1 month after the booster dose, the adverse reaction not already captured by solicited local and systemic reactions was injection site pain (n = 1; 4.2%).
Serious Adverse Events
No serious adverse events were reported in the 24 participants 6 through 23 months of age from the study vaccination through 1 month post vaccination.
Non-Serious Adverse Events
Non-serious adverse events in participants 6 through 23 months of age from the study vaccination through 1 month post vaccination were reported in 3 (12.5%) Pfizer-BioNTech COVID-19 Vaccine, Bivalent recipients. Non-serious adverse events considered related to vaccination by the study investigator were fatigue (n=1; 4.2%) and injection site pain (n=1; 4.2%).
Participants 2 Through 4 Years of Age Who Received a Booster Dose with Pfizer-BioNTech COVID-19 Vaccine, Bivalent (Original and Omicron BA.4/BA.5)
In a subset of Study 6, 36 participants 2 through 4 years of age previously vaccinated with a 3-dose primary series of Pfizer-BioNTech COVID-19 Vaccine (3 mcg modRNA) received a booster (fourth dose) with Pfizer-BioNTech COVID-19 Vaccine, Bivalent (Original and Omicron BA.4/BA.5) (3 mcg modRNA).
Participants received a booster (fourth dose) with Pfizer-BioNTech COVID-19 Vaccine, Bivalent 2.2 to 8.5 months after receiving their third dose with Pfizer-BioNTech COVID-19 Vaccine and had a median follow-up time of 1.9 months (range 1.6 to 2.3 months) up to a data cutoff date of November 25, 2022. Their median age was 2 years (range 2 through 4 years of age), 55.6% were male and 44.4% were female, 61.1% were White, 30.6% were Hispanic/Latino, 22.2% were multi-racial, 11.1% were Asian, and 5.6% were Black or African American.
Solicited Local and Systemic Adverse Reactions
Table 9 and Table 10 present the frequency and severity of reported solicited local reactions and systemic reactions, respectively, within 7 days of a booster (fourth dose) of Pfizer-BioNTech COVID-19 Vaccine, Bivalent.
The mean duration of pain at the injection site was 1.1 days (range 1 to 2 days), for redness 1.3 days (range 1 to 2 days), and for swelling 3 days for participants 2 through 4 years of age.
Table 9: Local Adverse Reactions, by Maximum Severity, Within 7 Days After a Booster (Fourth Dose) – Participants 2 through 4 Years of Age – Safety Population
| Note: Reactions were collected in the electronic diary (e-diary) and at unscheduled clinical assessments from Day 1 through Day 7 after the study vaccination. Reactions reported as adverse events in the case report form within 7 days after the study vaccination were also included in the analysis; the severity of these events is based on the grading scale in the adverse event section of the case report form. | |
|
|
|
Pfizer-BioNTech COVID-19 Vaccine, Bivalent (Original and Omicron BA.4/BA.5) 3 mcg modRNA N*=36 n† (%) |
|
|
Redness‡ | |
|
Any (≥0.5 cm) |
3 (8.3) |
|
Mild |
2 (5.6) |
|
Moderate |
1 (2.8) |
|
Swelling‡ | |
|
Any (≥0.5 cm) |
1 (2.8) |
|
Mild |
0 |
|
Moderate |
1 (2.8) |
|
Pain at the injection site§ | |
|
Any |
10 (27.8) |
|
Mild |
8 (22.2) |
|
Moderate |
2 (5.6) |
Table 10: Frequency and Percentages of Participants with Solicited Systemic Reactions, by Maximum Severity, Within 7 Days After a Booster (Fourth Dose) – Participants 2 through 4 Years of Age – Safety Population
| Note: Events and use of antipyretic or pain medication were collected in the electronic diary (e-diary) and at unscheduled clinical assessments from Day 1 through Day 7 after the study vaccination. Events reported as adverse events in the case report form within 7 days after the study vaccination were also included in the analysis; the severity of these events is based on the grading scale in the adverse event section of the case report form. | |
|
|
|
Pfizer-BioNTech COVID-19 Vaccine, Bivalent (Original and Omicron BA.4/BA.5) 3 mcg modRNA N*=36 n† (%) |
|
|
Fever |
|
|
≥38.0℃ |
0 |
|
Fatigue‡ |
|
|
Any |
11 (30.6) |
|
Mild |
6 (16.7) |
|
Moderate |
5 (13.9) |
|
Headache‡ |
|
|
Any |
1 (2.8) |
|
Mild |
1 (2.8) |
|
Chills‡ |
|
|
Any |
1 (2.8) |
|
Mild |
1 (2.8) |
|
Vomiting§ |
|
|
Any |
1 (2.8) |
|
Mild |
1 (2.8) |
|
Diarrhea¶ |
|
|
Any |
2 (5.6) |
|
Mild |
1 (2.8) |
|
Moderate |
1 (2.8) |
|
New or worsened muscle pain‡ |
|
|
Any |
0 |
|
New or worsened joint pain‡ |
|
|
Any |
1 (2.8) |
|
Mild |
1 (2.8) |
|
Use of antipyretic or pain medication# |
1 (2.8) |
Unsolicited Adverse Events
Participants 2 through 4 years of age who received a booster (fourth dose) with the Pfizer-BioNTech COVID-19 Vaccine, Bivalent had a median follow-up time of 1.9 months (range 1.6 to 2.3 months) to the cutoff date (November 25, 2022).
Serious Adverse Events
No serious adverse events were reported in the 36 participants 2 through 4 years of age from the study vaccination through 1 month post vaccination.
Participants 5 Through 11 Years of Age Who Received a Booster Dose with Pfizer-BioNTech COVID-19 Vaccine, Bivalent (Original and Omicron BA.4/BA.5)
In Study 6, 113 participants 5 through 11 years of age previously vaccinated with a 2-dose primary series and 1 booster dose of Pfizer-BioNTech COVID-19 Vaccine (10 mcg modRNA) received a booster (fourth dose) with Pfizer-BioNTech COVID-19 Vaccine, Bivalent (Original and Omicron BA.4/BA.5) (10 mcg modRNA).
Participants received a booster (fourth dose) with Pfizer-BioNTech COVID-19, Bivalent 2.6 to 8.5 months after receiving their third dose with Pfizer-BioNTech COVID-19 Vaccine and had a median follow-up time of 1.6 months (range 1.1 to 2.3 months) up to a data cutoff date of November 25, 2022. Their median age was 9 years (range 5 through 11 years of age), 50.4% were male and 49.6% were female, 58.4% were White, 20.4% were Hispanic/Latino, 19.5% were multi-racial, 11.5% were Asian, and 8.0% were Black or African American.
Unsolicited Adverse Events
In the following analysis of Study 6, 113 participants 5 through 11 years of age who received a booster (fourth dose) with the Pfizer-BioNTech COVID-19 Vaccine, Bivalent had a median follow-up time of 1.6 months (range 1.1 to 2.3 months) to the cutoff date (November 25, 2022).
Serious Adverse Events
No serious adverse events were reported in the 113 participants 5 through 11 years of age from the study vaccination through 1 month post vaccination.
Non-Serious Adverse Events
Lymphadenopathy 2 days post-vaccination, considered related to vaccination, was reported in 1 (0.9%) participant 5 through 11 years of age.
Participants 12 Years of Age and Older Who Received a Booster Dose with Pfizer-BioNTech COVID-19 Vaccine, Bivalent (Original and Omicron BA.4/BA.5)
A subset of Study 5 Phase 2/3 participants 12 through 17 years of age (n=107), 18 through 55 years of age (n=103) and 56 years of age and older (n=106) previously vaccinated with a 2-dose primary series and 1 booster dose of Pfizer-BioNTech COVID-19 Vaccine (30 mcg modRNA), received a second booster dose with Pfizer-BioNTech COVID-19 Vaccine, Bivalent (Original and Omicron BA.4/BA.5) (30 mcg modRNA).
The participants received the second booster dose a median of 9.9 months (range 5.5 to 14.3 months) after receiving the first booster dose and had a median follow-up time of 1.6 months up to a data cutoff date of October 12, 2022. The median age was 40.0 years, 53.2% were male, 46.8% were female, 81.3% were White, 9.2% were Hispanic/Latino, 5.1% were Asian, and 10.8% were Black or African American.
Unsolicited Adverse Events
In the following analysis of Study 5, 316 participants 12 years of age and older who received a second booster of Pfizer-BioNTech COVID-19 Vaccine, Bivalent had a median follow-up time of 1.6 months (range 1.3 to 1.8 months) to the cutoff date October 12, 2022.
Serious Adverse Events
Serious adverse events were reported in the 1 participant (considered unrelated to the vaccine) from the study vaccination through 1 month post vaccination.
Non-Serious Adverse Events
Lymphadenopathy 2 days post-vaccination, considered related to vaccination, was reported in 1 (0.3%) participant 12 years of age and older.
6.2 Post Authorization Experience
The following adverse reactions have been identified during post authorization use of Pfizer-BioNTech COVID-19 Vaccine. Because these reactions are reported voluntarily, it is not always possible to reliably estimate their frequency or establish a causal relationship to vaccine exposure.
Cardiac Disorders: myocarditis, pericarditis
Gastrointestinal Disorders: diarrhea, vomiting
Immune System Disorders: severe allergic reactions, including anaphylaxis, and other hypersensitivity reactions (e.g., rash, pruritus, urticaria, angioedema)
Musculoskeletal and Connective Tissue Disorders: pain in extremity (arm)
Nervous System Disorders: syncope, dizziness
8 REQUIREMENTS AND INSTRUCTIONS FOR REPORTING ADVERSE EVENTS AND VACCINE ADMINISTRATION ERRORS
See Overall Safety Summary (Section 6) for additional information.
The vaccination provider enrolled in the federal COVID-19 Vaccination Program is responsible for MANDATORY reporting of the listed events following Pfizer-BioNTech COVID-19 Vaccine or Pfizer-BioNTech COVID-19 Vaccine, Bivalent to the Vaccine Adverse Event Reporting System (VAERS):
- •
- Vaccine administration errors whether or not associated with an adverse event
- •
- Serious adverse events* (irrespective of attribution to vaccination)
- •
- Cases of myocarditis
- •
- Cases of pericarditis
- •
- Cases of Multisystem Inflammatory Syndrome (MIS) in children and adults
- •
- Cases of COVID-19 that result in hospitalization or death
*Serious adverse events are defined as:
- •
- Death
- •
- A life-threatening adverse event
- •
- Inpatient hospitalization or prolongation of existing hospitalization
- •
- A persistent or significant incapacity or substantial disruption of the ability to conduct normal life functions
- •
- A congenital anomaly/birth defect
- •
- An important medical event that based on appropriate medical judgement may jeopardize the individual and may require medical or surgical intervention to prevent 1 of the outcomes listed above
Instructions for Reporting to VAERS
The vaccination provider enrolled in the federal COVID-19 Vaccination Program should complete and submit a VAERS form to FDA using 1 of the following methods:
- •
- Complete and submit the report online: https://vaers.hhs.gov/reportevent.html, or
- •
- If you are unable to submit this form electronically, you may fax it to VAERS at 1-877-721-0366. If you need additional help submitting a report you may call the VAERS toll-free information line at 1-800-822-7967 or send an email to info@vaers.org.
IMPORTANT: When reporting adverse events or vaccine administration errors to VAERS, please complete the entire form with detailed information. It is important that the information reported to FDA be as detailed and complete as possible. Information to include:
- •
- Patient demographics (e.g., patient name, date of birth)
- •
- Pertinent medical history
- •
- Pertinent details regarding admission and course of illness
- •
- Concomitant medications
- •
- Timing of adverse event(s) in relationship to administration of the Pfizer-BioNTech COVID-19 Vaccine
- •
- Pertinent laboratory and virology information
- •
- Outcome of the event and any additional follow-up information if it is available at the time of the VAERS report. Subsequent reporting of follow-up information should be completed if additional details become available.
The following steps are highlighted to provide the necessary information for safety tracking:
- 1.
- In Box 17, provide information on Pfizer-BioNTech COVID-19 Vaccine or Pfizer-BioNTech COVID-19 Vaccine, Bivalent and any other vaccines administered on the same day; and in Box 22, provide information on any other vaccines received within 1 month prior.
- 2.
- In Box 18, description of the event:
- a.
- Write "Pfizer-BioNTech COVID-19 Vaccine EUA" or "Pfizer-BioNTech COVID-19 Vaccine, Bivalent EUA" as the first line.
- b.
- Provide a detailed report of vaccine administration error and/or adverse event. It is important to provide detailed information regarding the patient and adverse event/medication error for ongoing safety evaluation of this unapproved vaccine. Please see information to include listed above.
- 3.
- Contact information:
- c.
- In Box 13, provide the name and contact information of the prescribing healthcare provider or institutional designee who is responsible for the report.
- d.
- In Box 14, provide the name and contact information of the best doctor/healthcare professional to contact about the adverse event.
- e.
- In Box 15, provide the address of the facility where vaccine was given (NOT the healthcare provider's office address).
Other Reporting Instructions
Vaccination providers may report to VAERS other adverse events that are not required to be reported using the contact information above.
To the extent feasible, report adverse events to Pfizer Inc. using the contact information below or by providing a copy of the VAERS form to Pfizer Inc.
| Website | Fax number | Telephone number |
|---|---|---|
|
1-866-635-8337 |
1-800-438-1985 |
10 DRUG INTERACTIONS
There are no data to assess the concomitant administration of the Pfizer-BioNTech COVID-19 Vaccine or the Pfizer-BioNTech COVID-19 Vaccine, Bivalent with other vaccines.
11 USE IN SPECIFIC POPULATIONS
11.3 Pediatric Use
Pfizer-BioNTech COVID-19 Vaccine is authorized for use in individuals 6 months through 17 years of age. This authorization is based on safety and effectiveness data in this age group and adults.
Pfizer-BioNTech COVID-19 Vaccine is not authorized for use in individuals younger than 6 months of age.
Pfizer-BioNTech COVID-19 Vaccine, Bivalent is authorized for use in individuals 6 months through 17 years of age. This authorization is based on the safety and effectiveness data of Pfizer-BioNTech COVID-19 Vaccine in individuals 6 months of age and older and safety and immunogenicity data with the bivalent vaccine (Original and Omicron BA.1) in individuals greater than 55 years of age and Pfizer-BioNTech COVID-19 Vaccine, Bivalent in individuals 6 months of age and older.
Pfizer-BioNTech COVID-19 Vaccine, Bivalent is not authorized for use in individuals younger than 6 months of age.
13 DESCRIPTION
The Pfizer-BioNTech COVID-19 Vaccine in multiple dose vials with maroon caps and labels with maroon borders is supplied as a frozen suspension; each vial must be diluted with 2.2 mL of sterile 0.9% Sodium Chloride Injection, USP prior to use to form the vaccine.
Each 0.2 mL dose of the Pfizer-BioNTech COVID-19 Vaccine supplied in multiple dose vials with maroon caps and labels with maroon borders is formulated to contain 3 mcg of modRNA encoding the spike (S) glycoprotein of the SARS-CoV-2 Wuhan-Hu-1 strain (Original).
The Pfizer-BioNTech COVID-19 Vaccine, Bivalent in multiple dose vials with maroon caps and labels with maroon borders is supplied as a frozen suspension; each vial must be diluted with 2.2 mL of sterile 0.9% Sodium Chloride Injection, USP prior to use to form the vaccine.
Each 0.2 mL dose of the Pfizer-BioNTech COVID-19 Vaccine, Bivalent supplied in multiple dose vials with maroon caps and labels with maroon borders is formulated to contain 1.5 mcg of modRNA encoding the S-glycoprotein of SARS-CoV-2 Wuhan-Hu-1 strain (Original) and 1.5 mcg of modRNA encoding the S-glycoprotein of SARS-CoV-2 Omicron variant lineages BA.4 and BA.5 (Omicron BA.4/BA.5). The S-glycoproteins of the SARS-CoV-2 Omicron variant lineages BA.4 and BA.5 are identical. Each dose contains 3 mcg modRNA.
Each 0.2 mL dose of the Pfizer-BioNTech COVID-19 Vaccine and the Pfizer-BioNTech COVID-19 Vaccine, Bivalent supplied in multiple dose vials with maroon caps and labels with maroon borders also includes the following ingredients: lipids (0.04 mg ((4-hydroxybutyl)azanediyl)bis(hexane-6,1-diyl)bis(2-hexyldecanoate), 0.005 mg 2[(polyethylene glycol)-2000]-N,N-ditetradecylacetamide, 0.01 mg 1,2-distearoyl-sn-glycero-3-phosphocholine, and 0.02 mg cholesterol), 3.2 mg sucrose, 0.006 mg tromethamine, and 0.04 mg tromethamine hydrochloride. The diluent (sterile 0.9% Sodium Chloride Injection, USP) contributes 1.52 mg sodium chloride per dose.
The Pfizer-BioNTech COVID-19 Vaccine and the Pfizer-BioNTech COVID-19 Vaccine, Bivalent do not contain preservative. The vial stoppers are not made with natural rubber latex.
14 CLINICAL PHARMACOLOGY
14.1 Mechanism of Action
The modRNA in the Pfizer-BioNTech COVID-19 Vaccine and the Pfizer-BioNTech COVID-19 Vaccine, Bivalent are formulated in lipid particles, which enable delivery of the RNA into host cells to allow expression of the SARS-CoV-2 S antigen. The vaccine elicits an immune response to the S antigen, which protects against COVID-19.
18 CLINICAL TRIAL RESULTS AND SUPPORTING DATA FOR EUA
The effectiveness of the Pfizer-BioNTech COVID-19 Vaccine for individuals 6 months through 4 years of age is based on efficacy of primary vaccination with this vaccine in individuals 16 years of age and older and effectiveness of primary vaccination with this vaccine in individuals 6 months through 4 years of age.
The effectiveness of Pfizer-BioNTech COVID-19 Vaccine, Bivalent for the third dose of the primary series or for a booster dose in individuals 6 months through 4 years of age after completion of primary vaccination with 3 doses of the Pfizer-BioNTech COVID-19 Vaccine is based on:
- •
- efficacy of primary vaccination with Pfizer-BioNTech COVID-19 Vaccine in individuals 16 years of age and older,
- •
- effectiveness of primary vaccination with Pfizer-BioNTech COVID-19 Vaccine in individuals 6 months through 4 years of age,
- •
- immunogenicity of a booster dose with the bivalent vaccine (Original and Omicron BA.1) in individuals greater than 55 years of age, and
- •
- immunogenicity of a booster dose with Pfizer-BioNTech COVID-19 Vaccine, Bivalent in individuals 6 months through 4 years of age.
18.1 Efficacy of a 2-Dose Primary Series in Participants 16 Years of Age and Older
Study 2 is a multicenter, multinational, Phase 1/2/3, randomized, placebo-controlled, observer-blind, dose-finding, vaccine candidate-selection, and efficacy study in participants 12 years of age and older. Randomization was stratified by age: 12 through 15 years of age, 16 through 55 years of age, or 56 years of age and older, with a minimum of 40% of participants in the ≥56-year stratum. The study excluded participants who were immunocompromised and those who had previous clinical or microbiological diagnosis of COVID-19. Participants with preexisting stable disease, defined as disease not requiring significant change in therapy or hospitalization for worsening disease during the 6 weeks before enrollment, were included as were participants with known stable infection with human immunodeficiency virus (HIV), hepatitis C virus (HCV), or hepatitis B virus (HBV).
In the Phase 2/3 portion of Study 2, based on data accrued through November 14, 2020, approximately 44,000 participants 12 years of age and older were randomized equally and received 2 doses of Pfizer-BioNTech COVID-19 Vaccine (30 mcg modRNA) or placebo separated by 21 days. Participants are planned to be followed for up to 24 months, for assessments of safety and efficacy against COVID-19.
The population for the analysis of the primary efficacy endpoint included 36,621 participants 12 years of age and older (18,242 in the Pfizer-BioNTech COVID-19 Vaccine group and 18,379 in the placebo group) who did not have evidence of prior infection with SARS-CoV-2 through 7 days after the second dose. Table 11 presents the specific demographic characteristics in the studied population.
|
||
|
Pfizer-BioNTech
|
Placebo
|
|
|
Sex | ||
|
Male |
9318 (51.1) |
9225 (50.2) |
|
Female |
8924 (48.9) |
9154 (49.8) |
|
Age (years) | ||
|
Mean (SD) |
50.6 (15.70) |
50.4 (15.81) |
|
Median |
52.0 |
52.0 |
|
Min, max |
(12, 89) |
(12, 91) |
|
Age group | ||
|
≥12 through 15 years‡ |
46 (0.3) |
42 (0.2) |
|
≥16 through 17 years |
66 (0.4) |
68 (0.4) |
|
≥16 through 64 years |
14,216 (77.9) |
14,299 (77.8) |
|
≥65 through 74 years |
3176 (17.4) |
3226 (17.6) |
|
≥75 years |
804 (4.4) |
812 (4.4) |
|
Race | ||
|
White |
15,110 (82.8) |
15,301 (83.3) |
|
Black or African American |
1617 (8.9) |
1617 (8.8) |
|
American Indian or Alaska Native |
118 (0.6) |
106 (0.6) |
|
Asian |
815 (4.5) |
810 (4.4) |
|
Native Hawaiian or other Pacific Islander |
48 (0.3) |
29 (0.2) |
|
Other§ |
534 (2.9) |
516 (2.8) |
|
Ethnicity | ||
|
Hispanic or Latino |
4886 (26.8) |
4857 (26.4) |
|
Not Hispanic or Latino |
13,253 (72.7) |
13,412 (73.0) |
|
Not reported |
103 (0.6) |
110 (0.6) |
|
Comorbidities¶ | ||
|
Yes |
8432 (46.2) |
8450 (46.0) |
|
No |
9810 (53.8) |
9929 (54.0) |
The population in the primary efficacy analysis included all participants 12 years of age and older who had been enrolled from July 27, 2020, and followed for the development of COVID-19 through November 14, 2020. Participants 18 through 55 years of age and 56 years of age and older began enrollment from July 27, 2020, 16 through 17 years of age began enrollment from September 16, 2020, and 12 through 15 years of age began enrollment from October 15, 2020.
The vaccine efficacy information is presented in Table 12.
| Note: Confirmed cases were determined by Reverse Transcription-Polymerase Chain Reaction (RT-PCR) and at least 1 symptom consistent with COVID-19 (symptoms included: fever; new or increased cough; new or increased shortness of breath; chills; new or increased muscle pain; new loss of taste or smell; sore throat; diarrhea; vomiting). | |||
|
|||
|
First COVID-19 occurrence from 7 days after Dose 2 in participants without evidence of prior SARS-CoV-2 infection* |
|||
|
Subgroup |
Pfizer-BioNTech COVID-19 Vaccine†
|
Vaccine Efficacy %
|
|
|
All participantsÞ |
8 |
162 |
95.0 |
|
16 through 64 years |
7 |
143 |
95.1 |
|
65 years and older |
1 |
19 |
94.7 |
|
First COVID-19 occurrence from 7 days after Dose 2 in participants with or without evidence of prior SARS-CoV-2 infection |
|||
|
Subgroup |
Pfizer-BioNTech COVID-19 Vaccine†
|
Vaccine Efficacy %
|
|
|
All participantsÞ |
9 |
169 |
94.6 |
|
16 through 64 years |
8 |
150 |
94.6 |
|
65 years and older |
1 |
19 |
94.7 |
18.2 Effectiveness of a 3-Dose Primary Series in Participants 6 Months Through 4 Years of Age
Study 3 is an ongoing Phase 1/2/3 multicenter, randomized, dose finding, open label (Phase 1) and multinational, saline placebo-controlled, observer-blind, immunogenicity and efficacy (Phase 2/3) study to evaluate the safety and effectiveness of Pfizer-BioNTech COVID-19 Vaccine in individuals 6 months through 11 years of age. Randomization was stratified by age: 6 through 23 months of age, 2 through 4 years of age, or 5 through 11 years of age. The study excluded participants who were immunocompromised and those who had previous clinical or microbiological diagnosis of COVID-19. Results from participants 6 months through 4 years of age are presented in this subsection. In Phase 2/3, a total of 1,776 participants 6 through 23 months of age and 2,750 participants 2 through 4 years of age were randomized 2:1 and received 3 doses of the Pfizer-BioNTech COVID-19 Vaccine or saline placebo.
Effectiveness in individuals 6 months through 4 years of age is based on a comparison of immune responses in this age group to individuals 16 through 25 years of age.
Immunogenicity in Participants 2 Through 4 Years of Age After a 3-Dose Primary Series
Immunogenicity analyses have been performed in the immunobridging subset of 143 Study 3 participants 2 through 4 years of age without evidence of infection up to 1 month after Dose 3 based on a data cutoff date of April 29, 2022.
The evaluable immunogenicity population without prior evidence of SARS-CoV-2 infection up to 1 month after Dose 3 of Pfizer-BioNTech COVID-19 Vaccine was comprised of 143 participants 2 through 4 years of age. Most participants in this analysis population were White (69.2%), with 5.6% Black or African American participants, 11.2% Asian participants, and 11.9% multi-racial participants. There were 11.2% Hispanic/Latino participants. The median age was 3.0 years and 44.1% of participants were male. There were 6.3% of participants reported as obese. In the evaluable immunogenicity population (regardless of evidence of prior infection), 11/204 participants (5.4%) were baseline positive for prior SARS-CoV-2 infection.
SARS-CoV-2 50% neutralizing antibody titers (NT50) were compared between an immunogenicity subset of Phase 2/3 participants 2 through 4 years of age from Study 3 at 1 month after the 3-dose primary series and a randomly selected subset from Study 2 Phase 2/3 participants 16 through 25 years of age at 1 month after the 2-dose primary series, using a microneutralization assay against the reference strain (USA_WA1/2020). The primary immunobridging analyses compared the geometric mean titers (using a geometric mean ratio [GMR]) and the seroresponse (defined as achieving at least 4-fold rise in SARS-CoV-2 NT50 from before Dose 1) rates in the evaluable immunogenicity population of participants without evidence of prior SARS-CoV-2 infection up to 1 month after Dose 3 in participants 2 through 4 years of age and up to 1 month after Dose 2 in participants 16 through 25 years of age. The prespecified immunobridging criteria were met for both the GMR and the seroresponse difference (Table 13 and Table 14, respectively).
| Abbreviations: CI = confidence interval; GMR = geometric mean ratio; GMT = geometric mean titer; LLOQ = lower limit of quantitation; NAAT = nucleic acid amplification test; NT50 = 50% neutralizing titer; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2. Note: Participants who had no serological or virological evidence [(up to 1 month after Dose 2 (Study 2) or 1 month after Dose 3 (Study 3) blood sample collection)] of past SARS-CoV-2 infection [(i.e., N-binding antibody [serum] negative at Dose 1, Dose 3 (Study 3) and 1 month after Dose 2 (Study 2) or 1 month after Dose 3 (Study 3), SARS-CoV-2 not detected by NAAT [nasal swab] at Dose 1, Dose 2, and Dose 3 (Study 3) study visits, and negative NAAT (nasal swab) at any unscheduled visit up to 1 month after Dose 2 (Study 2) or 1 month after Dose 3 (Study 3) blood collection)] and had no medical history of COVID-19 were included in the analysis. |
|||
|
|||
|
Pfizer-BioNTech COVID-19 Vaccine | |||
|
3 mcg modRNA/Dose
|
30 mcg modRNA /Dose
|
GMR (95%CI) |
|
|
Assay | |||
|
SARS-CoV-2 neutralization assay - NT50 (titer)¶ |
1535.2 |
1180.0 |
1.30 |
| Abbreviations: LLOQ = lower limit of quantitation; NAAT = nucleic acid amplification test; N-binding = SARS-CoV-2 nucleoprotein–binding; NT50 = 50% neutralizing titer 50; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2. Note: Seroresponse is defined as achieving a ≥4-fold rise from baseline (before Dose 1). If the baseline measurement is below the LLOQ, a postvaccination assay result ≥4 × LLOQ is considered a seroresponse. Note: Participants who had no serological or virological evidence (up to 1 month after Dose 2 [(Study 2) or 1 month after Dose 3 (Study 3) blood sample collection)[ of past SARS-CoV-2 infection [(i.e., N-binding antibody [serum] negative at pre-Dose 1, pre-Dose 3 (Study 3) and 1 month after Dose 2 (Study 2) or 1 month after Dose 3 (Study 3), SARS-CoV-2 not detected by NAAT [nasal swab] at pre-Dose 1, pre-Dose 2, and pre-Dose 3 (Study 3) study visits, and negative NAAT (nasal swab) at any unscheduled visit up to 1 month after Dose 2 (Study 2) or 1 month after Dose 3 (Study 3) blood collection)] and had no medical history of COVID-19 were included in the analysis. |
|||
|
|||
|
Pfizer-BioNTech COVID-19 Vaccine | |||
|
3 mcg modRNA/Dose
|
30 mcg modRNA/Dose
|
Difference in Seroresponse Rates %
|
|
|
Assay | |||
|
SARS-CoV-2 neutralization assay - NT50 (titer)# |
141 (100.0) |
168 (98.8) |
1.2 |
Using a non-validated fluorescence focus reduction neutralization test assay against the Omicron variant of SARS-CoV-2 (BA.1), the NT50 GMT at 1 month after Dose 3 among a subset of 34 study participants without evidence of prior SARS-CoV-2 infection (82.5 [95% confidence interval (CI): 55.4, 122.9]) was increased compared to the NT50 GMT before Dose 3 (14.0 [95% CI: 10.6, 18.5]).
Immunogenicity in Participants 6 Through 23 Months of Age After a 3-Dose Primary Series
Immunogenicity analyses have been performed in the immunobridging subset of 82 Study 3 participants 6 through 23 months of age without evidence of infection up to 1 month after Dose 3 based on a data cutoff date of April 29, 2022.
The evaluable immunogenicity population without prior evidence of SARS-CoV-2 infection up to 1 month after Dose 3 of Pfizer-BioNTech COVID-19 Vaccine was comprised of 82 participants 6 through 23 months of age. Most participants in this analysis population were White (72.0%), with 1.2% Black or African American participants, 13.4% Asian participants, and 12.2% multi-racial participants. There were 15.9% Hispanic/Latino participants. The median age was 16.0 months and 62.2% of participants were male. In the evaluable immunogenicity population (regardless of evidence of prior infection), 6/132 participants (4.5%) were baseline positive for prior SARS-CoV-2 infection.
SARS-CoV-2 NT50 1 month after the vaccination series were compared between an immunogenicity subset of Phase 2/3 participants 6 through 23 months of age from Study 3 and a randomly selected subset from Study 2 Phase 2/3 participants 16 through 25 years of age, using a microneutralization assay against the reference strain (USA_WA1/2020). The primary immunobridging analyses compared the geometric mean titers (using a GMR) and the seroresponse (defined as achieving at least 4-fold rise in SARS-CoV-2 NT50 from before Dose 1) rates in the evaluable immunogenicity population of participants without evidence of prior SARS-CoV-2 infection up to 1 month after Dose 3 in participants 6 through 23 months of age and up to 1 month after Dose 2 in participants 16 through 25 years of age. The prespecified immunobridging criteria were met for both the GMR and the seroresponse difference (Table 15 and Table 16, respectively).
| Abbreviations: CI = confidence interval; GMR = geometric mean ratio; GMT = geometric mean titer; LLOQ = lower limit of quantitation; NAAT = nucleic acid amplification test; NT50 = 50% neutralizing titer; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2. Note: Participants who had no serological or virological evidence [(up to 1 month after Dose 2 (Study 2) or 1 month after Dose 3 (Study 3) blood sample collection)] of past SARS-CoV-2 infection (i.e., N-binding antibody [serum] negative at Dose 1, Dose 3 (Study 3) and 1 month after Dose 2 (Study 2) or 1 month after Dose 3 (Study 3), SARS-CoV-2 not detected by NAAT [nasal swab] at Dose 1, Dose 2, and Dose 3 (Study 3) study visits, and negative NAAT (nasal swab) at any unscheduled visit up to 1 month after Dose 2 (Study 2) or 1 month after Dose 3 (Study 3) blood collection)] and had no medical history of COVID-19 were included in the analysis. |
|||
|
|||
|
Pfizer-BioNTech COVID-19 Vaccine |
GMR (95%CI)
|
||
|
3 mcg modRNA/Dose
|
30 mcg modRNA/Dose
|
||
|
Assay | |||
|
SARS-CoV-2 neutralization assay - NT50 (titer)¶ |
1406.5 |
1180.0 |
1.19 |
| Abbreviations: LLOQ = lower limit of quantitation; NAAT = nucleic acid amplification test; N-binding = SARS-CoV-2 nucleoprotein–binding; NT50 = 50% neutralizing titer 50; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2. Note: Seroresponse is defined as achieving a ≥4-fold rise from baseline (before Dose 1). If the baseline measurement is below the LLOQ, a postvaccination assay result ≥4 × LLOQ is considered a seroresponse. Note: Participants who had no serological or virological evidence [(up to 1 month after Dose 2 (Study 2) or 1 month after Dose 3 (Study 3) blood sample collection) of past SARS-CoV-2 infection (i.e., N-binding antibody [serum] negative at pre-Dose 1, Dose 3 (Study 3) and 1 month after Dose 2 (Study 2) or 1 month after Dose 3 (Study 3), SARS-CoV-2 not detected by NAAT [nasal swab] at pre-Dose 1, pre-Dose 2, and pre-Dose 3 (Study 3) study visits, and negative NAAT (nasal swab) at any unscheduled visit up to 1 month after Dose 2 (Study 2) or 1 month after Dose 3 (Study 3) blood collection)] and had no medical history of COVID-19 were included in the analysis. |
|||
|
|||
|
Pfizer-BioNTech COVID-19 Vaccine |
Difference in Seroresponse Rates %*
|
||
|
3 mcg modRNA/Dose 6 Through 23 months of Age
|
30 mcg modRNA/Dose
|
||
|
Assay | |||
|
SARS-CoV-2 neutralization assay - NT50 (titer)# |
80 (100.0) |
168 (98.8) |
1.2 |
Using a non-validated fluorescence focus reduction neutralization test assay against the Omicron variant of SARS-CoV-2 (BA.1), the NT50 GMT at 1 month after Dose 3 among a subset of 32 study participants without evidence of prior SARS-CoV-2 infection (127.5 [95% CI: 90.2, 180.1]) was increased compared to the NT50 GMT before Dose 3 (16.3 [95% CI: 12.8, 20.8]).
Efficacy in Participants 6 Months Through 4 Years of Age After a 3-Dose Primary Series
A descriptive efficacy analysis of Study 3 was performed across the combined population of participants 6 months through 4 years of age based on PCR-confirmed COVID-19 cases among 873 participants in the Pfizer-BioNTech COVID-19 Vaccine group and 381 participants in the placebo group (2:1 randomization) who received 3 doses of study intervention during the blinded follow-up period when the Omicron variant of SARS-CoV-2 (BA.2) was the predominant variant in circulation (data cutoff date of June 17, 2022).
The evaluable efficacy population without prior evidence of SARS-CoV-2 infection up to 7 days after Dose 3 of Pfizer-BioNTech COVID-19 Vaccine was comprised of 873 vaccine recipients and 381 placebo recipients 6 months through 4 years of age. Most vaccine recipients in this analysis population were White (76.3%), with 3.4% Black or African American participants, 10.0% Asian participants, and 10.1% who identified as multi-racial, other or not reported. There were 11.2% Hispanic/Latino vaccine recipients. Among the vaccine recipients, 51.1% were female. The median age was 16.0 months in vaccine recipients 6 through 23 months of age and the median age was 3.0 years in vaccine recipients 2 through 4 years of age. In the evaluable efficacy population, 8.7% of vaccine recipients had one or more comorbidities that increase the risk of severe COVID-19 as described in the Morbidity and Mortality Weekly Report (MMWR) 69(32);1081–8 and/or obesity (BMI ≥95th percentile) for participants 2 through 4 years of age. Between participants who received Pfizer-BioNTech COVID-19 Vaccine and those who received placebo, there were no notable differences in demographics.
The median dose interval between Dose 2 and Dose 3 was 13.4 weeks (range 8 to 33 weeks) among participants 6 through 23 months of age and 10 weeks (range 8 to 34 weeks) among participants 2 through 4 years of age who received Pfizer-BioNTech COVID-19 Vaccine. The median length of blinded follow-up for efficacy after Dose 3 was 1.7 months for participants 6 through 23 months of age and 2.1 months for participants 2 through 4 years of age in the Dose 3 Evaluable Efficacy Population who received Pfizer-BioNTech COVID-19 Vaccine or placebo.
The vaccine efficacy results after Dose 3 in participants 6 months through 4 years of age are presented in Table 17.
| Abbreviations: NAAT = nucleic acid amplification test; N-binding = SARS-CoV-2 nucleoprotein–binding; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2; VE = vaccine efficacy. Note: Confirmed cases were determined by Reverse Transcription-Polymerase Chain Reaction (RT-PCR) and at least 1 symptom consistent with COVID-19 (symptoms included: fever; new or increased cough; new or increased shortness of breath; chills; new or increased muscle pain; new loss of taste or smell; sore throat; diarrhea; vomiting; inability to eat/poor feeding). |
|||
|
|||
|
First COVID-19 occurrence from 7 days after Dose 3 in participants without evidence of prior SARS-CoV-2 infection* |
|||
|
Subgroup |
Pfizer-BioNTech COVID-19 Vaccine
|
Vaccine Efficacy %
|
|
|
6 months through 4 years# |
13 |
21 |
73.2 |
|
2 through 4 years |
9 |
13 |
71.8 |
|
6 through 23 months |
4 |
8 |
75.8 |
|
First COVID-19 occurrence from 7 days after Dose 3 in participants with or without evidence of prior SARS-CoV-2 infection |
|||
|
Subgroup |
Pfizer-BioNTech COVID-19 Vaccine
|
Vaccine Efficacy %
|
|
|
6 months through 4 years# |
14 |
23 |
72.5 |
|
2 through 4 years |
10 |
15 |
70.7 |
|
6 through 23 months |
4 |
8 |
76.2 |
Among participants 6 months through 4 years of age, severe COVID-19 case criteria were fulfilled after Dose 3 in 1 placebo recipient in the 6 through 23-month age group. This case occurred 44 days after Dose 3, based on a single criterion (increased heart rate) and did not require hospitalization. There were no cases of multisystem inflammatory syndrome in children reported through the June 17, 2022 data cutoff date.
18.3 Immunogenicity of Pfizer-BioNTech COVID-19 Vaccine, Bivalent (Original and Omicron BA.4/BA.5) Administered as a Booster (Fourth Dose) in Individuals 6 Months Through 4 Years of Age
In Study 6, a subset of 60 participants 6 months through 4 years of age received a booster dose (fourth dose) of Pfizer-BioNTech COVID-19 Vaccine, Bivalent (3 mcg modRNA) after receiving 3 prior doses of Pfizer-BioNTech COVID-19 Vaccine (3 mcg modRNA). Neutralizing antibody levels following the fourth dose are presented in Table 18. Data from a subset of participants 6 months through 4 years of age in Study 3 who received 3 doses of Pfizer-BioNTech COVID-19 Vaccine (3 mcg modRNA) are included as a reference. There were no formal statistical comparisons of the immune response between subsets from the two studies.
| Abbreviations: GMT = geometric mean titer; LLOQ = lower limit of quantitation; N-binding = SARS-CoV-2 nucleoprotein-binding; NAAT = nucleic acid amplification test. NT50 = 50% neutralizing titer; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2. |
||||||
|
||||||
|
SARS-CoV-2 neutralization Assay |
Age Group |
Sampling Time Point† |
Study 6 Pfizer-BioNTech COVID‑19 Vaccine, Bivalent (Original/Omicron BA.4/BA.5) 3 mcg modRNA Dose 4 and 1 Month After Dose 4 |
Study 3 Pfizer-BioNTech COVID‑19 Vaccine 3 mcg modRNA Dose 3 and 1 Month After Dose 3 |
||
|
n‡ |
n‡ | |||||
|
Omicron BA.4/BA.5 - NT50 (titer)¶ |
6 through 23 months |
Pre- vaccination |
21 |
243.9 (115.3, 516.1) |
23 |
96.0 (55.3, 166.8) |
|
1 month |
23 |
2011.4 (1141.3, 3544.9) |
23 |
625.6 (365.7, 1070.5) |
||
|
2 through 4 years |
Pre- vaccination |
33 |
165.6 (88.3, 310.5) |
31 |
56.1 (38.0, 82.7) |
|
|
1 month |
35 |
1514.9 (882.2, 2601.5) |
31 |
595.0 (370.5, 955.6) |
||
|
Reference strain - NT50 (titer)¶ |
6 through 23 months |
Pre-vaccination |
22 |
2491.2 (1432.0, 4333.8) |
22 |
981.6 (503.5, 1913.7) |
|
1 month |
23 |
8737.2 (5959.6, 12809.5) |
23 |
9221.7 (6734.0, 12628.3) |
||
|
2 through |
Pre-vaccination |
35 |
2802.7 (1795.7, 4374.3) |
31 |
657.9 (421.5, 1026.9) |
|
|
1 month |
35 |
10448.3 (7685.1, 14205.1) |
30 |
8933.3 (6388.0, 12492.9) |
||
18.4 Immunogenicity of the Bivalent Vaccine (Original and Omicron BA.1) Administered as a Second Booster Dose in Individuals Greater than 55 Years of Age
In an analysis of a subset from Study 4, a total of 610 participants greater than 55 years of age who had previously received a 2-dose primary series and 1 booster dose with Pfizer-BioNTech COVID-19 Vaccine received 1 of the following as a second booster dose: Pfizer-BioNTech COVID-19 Vaccine or bivalent vaccine (Original and Omicron BA.1). GMRs and seroresponse rates were evaluated at 1 month after vaccination with the bivalent vaccine (Original and Omicron BA.1). The bivalent vaccine (Original and Omicron BA.1) booster dose was administered 4.7 to 11.5 months (median 6.3 months) after the first booster dose. The Pfizer-BioNTech COVID-19 Vaccine booster dose was administered 5.3 to 13.1 months (median 6.3 months) after the first booster dose.
The primary objective of the study was to assess superiority with respect to level of 50% neutralizing titer (NT50) and noninferiority with respect to seroresponse rate of the anti-Omicron BA.1 immune response induced by a dose of the bivalent vaccine (Original and Omicron BA.1) relative to the response elicited by a dose of Pfizer-BioNTech COVID-19 Vaccine given as a second booster dose in participants greater than 55 years of age.
A secondary objective of the study was to assess noninferiority with respect to level of NT50 to the Original SARS-COV-2 strain induced by a dose of the bivalent vaccine (Original and Omicron BA.1) relative to the response elicited by a dose of Pfizer-BioNTech COVID-19 Vaccine given as a second booster dose. A comparison of seroresponse rates to the Original strain was descriptive.
Superiority of the anti-Omicron BA.1 NT50 for the bivalent vaccine (Original and Omicron BA.1) relative to Pfizer-BioNTech COVID-19 Vaccine was met, as the lower bound of the 2-sided 95% CI for GMR was >1. Noninferiority of the anti-Original NT50 for the bivalent vaccine (Original and Omicron BA.1) relative to Pfizer-BioNTech COVID-19 Vaccine was met, as the lower bound of the 2-sided 95% CI for GMR was >0.67 and the point estimate of the GMR was ≥0.8 (Table 19).
Noninferiority of the seroresponse rate to the Omicron BA.1 variant for the bivalent vaccine (Original and Omicron BA.1) relative to Pfizer-BioNTech COVID-19 Vaccine was met as the lower limit of the 2-sided 95% CI for the difference in percentages of participants with seroresponse is >-5% (Table 20). A descriptive summary of seroresponse to the Original strain is also included in Table 20.
| Abbreviations: GMR = geometric mean ratio; GMT = geometric mean titer; LLOQ = lower limit of quantitation; N-binding = SARS-CoV-2 nucleoprotein–binding; NAAT = nucleic acid amplification test; NT50 = 50% neutralizing titer; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2. Note: Immunogenicity subset = a random sample of 230 participants in each vaccine group. Note: Participants who had no serological or virological evidence (prior to the 1-month post–study vaccination blood sample collection) of past SARS-CoV-2 infection (i.e., N-binding antibody [serum] result negative at the study vaccination and the 1-month post–study vaccination visits, negative NAAT [nasal swab] result at the study vaccination visit, and any unscheduled visit prior to the 1-month post–study vaccination blood sample collection) and had no medical history of COVID-19 were included in the analysis. |
|||||
|
|||||
|
Assay |
Vaccine Group
|
Sampling Time Point* |
N† |
GMT
|
GMR
|
|
SARS-CoV-2 neutralization assay - Omicron BA.1 - NT50 (titer)¶ |
Pfizer-BioNTech COVID-19 Vaccine |
1 month |
163 |
455.8 | |
|
Bivalent Vaccine (Original and Omicron BA.1) |
1 month |
178 |
711.0 |
1.56 |
|
|
SARS-CoV-2 neutralization assay - Original strain - NT50 (titer)¶ |
Pfizer-BioNTech COVID-19 Vaccine |
1 month |
182 |
5998.1 | |
|
Bivalent Vaccine (Original and Omicron BA.1) |
1 month |
186 |
5933.2 |
0.99 |
|
| Abbreviations: LLOQ = lower limit of quantitation; N-binding = SARS-CoV-2 nucleoprotein–binding; NAAT = nucleic acid amplification test; NT50 = 50% neutralizing titer; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2. Note: Immunogenicity subset = a random sample of 230 participants in each vaccine group. Note: Seroresponse is defined as achieving ≥ 4-fold rise from baseline (before the second booster dose). If the baseline measurement is below the LLOQ, the postvaccination measure of ≥ 4 × LLOQ is considered a seroresponse. Note: Participants who had no serological or virological evidence (prior to the 1-month post–study vaccination blood sample collection) of past SARS-CoV-2 infection (i.e., N-binding antibody [serum] result negative at the study vaccination and the 1-month post–study vaccination visits, negative NAAT [nasal swab] result at the study vaccination visit, and any unscheduled visit prior to the 1-month post–study vaccination blood sample collection) and had no medical history of COVID-19 were included in the analysis. |
|||||
|
|||||
|
Assay |
Vaccine Group
|
Sampling Time Point* |
N† | ||
|
SARS-CoV-2 neutralization assay - Omicron BA.1 - NT50 (titer)Þ |
Pfizer-BioNTech COVID-19 Vaccine |
1 month |
149 |
85 (57.0) | |
|
Bivalent Vaccine (Original and Omicron BA.1) |
1 month |
169 |
121 (71.6) |
14.6 |
|
|
SARS-CoV-2 neutralization assay - Original strain - NT50 (titer)Þ |
Pfizer-BioNTech COVID-19 Vaccine |
1 month |
179 |
88 (49.2) | |
|
Bivalent Vaccine (Original and Omicron BA.1) |
1 month |
186 |
93 (50.0) | ||
19 HOW SUPPLIED/STORAGE AND HANDLING
The information in this section applies to the Pfizer-BioNTech COVID-19 Vaccine and the Pfizer-BioNTech COVID-19 Vaccine, Bivalent that are supplied in multiple dose vials with maroon caps and labels with maroon borders. After dilution, 1 vial contains 10 doses of 0.2 mL.
Pfizer-BioNTech COVID-19 Vaccine:
Multiple dose vials are supplied in a carton containing 10 multiple dose vials.
- •
- Carton of 10 multiple dose vials: NDC 59267-0078-4
- •
- Multiple dose vial: NDC 59267-0078-1
Pfizer-BioNTech COVID-19 Vaccine, Bivalent:
Multiple dose vials are supplied in a carton containing 10 multiple dose vials.
- •
- Carton of 10 multiple dose vials: NDC 59267-0609-2
- •
- Multiple dose vial: NDC 59267-0609-1
During storage, minimize exposure to room light, and avoid exposure to direct sunlight and ultraviolet light.
Do not refreeze thawed vials.
Vial Storage Prior to Use
Cartons of Pfizer-BioNTech COVID-19 Vaccine and Pfizer-BioNTech COVID-19 Vaccine, Bivalent multiple dose vials with maroon caps and labels with maroon borders may arrive frozen at ultra-cold conditions in thermal containers with dry ice.
Once received, frozen vials may be immediately transferred to the refrigerator [2°C to 8°C (35°F to 46°F)], thawed and stored for up to 10 weeks. The 10-week refrigerated expiry date should be recorded on the carton at the time of transfer. A carton of 10 vials may take up to 2 hours to thaw at this temperature.
Alternatively, frozen vials may be stored in an ultra-low temperature freezer at -90°C to -60°C (-130°F to -76°F) for up to 18 months from the date of manufacture. Do not store vials at -25°C to -15°C (-13°F to 5°F). Once vials are thawed, they should not be refrozen.
If cartons of Pfizer-BioNTech COVID-19 Vaccine and Pfizer-BioNTech COVID-19 Vaccine, Bivalent multiple dose vials with maroon caps and labels with maroon borders are received at 2°C to 8°C (35°F to 46°F), they should be stored at 2°C to 8°C (35°F to 46°F). Check that the carton has been updated to reflect the 10-week refrigerated expiry date.
Regardless of storage condition, the vaccine should not be used after 18 months from the date of manufacture printed on the vial and cartons.
Examples of expiry dates based on 18 months from the date of the manufacture for the Pfizer-BioNTech COVID-19 Vaccine or the Pfizer-BioNTech COVID-19 Vaccine, Bivalent are shown below.
| Printed Manufacturing Date | 18-Month Expiry Date | |
|---|---|---|
|
01/2022 |
30-Jun-2023 |
|
|
02/2022 |
31-Jul-2023 |
|
|
03/2022 |
31-Aug-2023 |
|
|
04/2022 |
30-Sep-2023 |
|
|
05/2022 |
31-Oct-2023 |
|
|
06/2022 |
30-Nov-2023 |
Vial Storage During Use
If not previously thawed at 2°C to 8°C (35°F to 46°F), allow vials to thaw at room temperature [up to 25°C (77°F)] for 30 minutes.
Pfizer-BioNTech COVID-19 Vaccine multiple dose vials with maroon caps and labels with maroon borders may be stored at room temperature [8°C to 25°C (46°F to 77°F)] for a total of 12 hours prior to dilution.
After dilution, the vial should be held between 2°C to 25°C (35°F to 77°F). Vials should be discarded 12 hours after dilution.
Pfizer-BioNTech COVID-19 Vaccine vial labels and cartons may state that a vial should be discarded 6 hours after the first puncture. The information in this Full EUA Prescribing Information supersedes the number of hours printed on vial labels and cartons.
20 PATIENT COUNSELING INFORMATION
Advise the caregiver to read the Fact Sheet for Recipients and Caregivers.
The vaccination provider must include vaccination information in the state/local jurisdiction's Immunization Information System (IIS) or other designated system. Advise recipient or caregiver that more information about IISs can be found at: https://www.cdc.gov/vaccines/programs/iis/about.html.
21 CONTACT INFORMATION
For general questions, visit the website or call the telephone number provided below.
| Website | Telephone number |
|---|---|
|
1-877-829-2619 |
This Full EUA Prescribing Information may have been updated. For the most recent Full EUA Prescribing Information, please see www.cvdvaccine.com.

Manufactured for
BioNTech Manufacturing GmbH
An der Goldgrube 12
55131 Mainz, Germany

Manufactured by
Pfizer Inc., New York, NY 10001
LAB-1515-5.0
Revised: 14 March 2023
FACT SHEET FOR RECIPIENTS AND CAREGIVERS ABOUT THE PFIZER-BIONTECH COVID-19 VACCINE AND THE PFIZER-BIONTECH COVID-19 VACCINE, BIVALENT (ORIGINAL AND OMICRON BA.4/BA.5) TO PREVENT CORONAVIRUS DISEASE 2019 (COVID-19) FOR USE IN INDIVIDUALS 6 MONTHS THROUGH 4 YEARS OF AGE
|
FOR 6 MONTHS THROUGH 4 YEARS OF AGE |
Your child is being offered the Pfizer-BioNTech COVID-19 Vaccine6 or the Pfizer-BioNTech COVID-19 Vaccine, Bivalent7 to prevent Coronavirus Disease 2019 (COVID-19) caused by SARS-CoV-2.
Primary Series
The Pfizer-BioNTech COVID-19 Vaccine and the Pfizer-BioNTech COVID-19 Vaccine, Bivalent have received Emergency Use Authorization (EUA) from the U.S. Food and Drug Administration (FDA) to provide a 3-dose primary series to individuals 6 months through 4 years of age as follows:8,9
- Dose 1: Pfizer-BioNTech COVID-19 Vaccine
- Dose 2: Pfizer-BioNTech COVID-19 Vaccine
- Dose 3: Pfizer-BioNTech COVID-19 Vaccine, Bivalent
Booster Dose
The Pfizer-BioNTech COVID-19 Vaccine, Bivalent is authorized for use in individuals 6 months through 4 years of age to provide a single booster dose given at least 2 months after completion of primary vaccination with 3 doses of the Pfizer-BioNTech COVID-19 Vaccine10 .
This Fact Sheet contains information to help you understand the risks and benefits of the Pfizer-BioNTech COVID-19 Vaccine and the Pfizer-BioNTech COVID-19 Vaccine, Bivalent, which your child may receive because there is currently a pandemic of COVID-19. Talk to your child's vaccination provider if you have questions.
This Fact Sheet may have been updated. For the most recent Fact Sheet, please see www.cvdvaccine.com.
WHAT YOU NEED TO KNOW BEFORE YOUR CHILD GETS ANY OF THESE VACCINES
WHAT IS COVID-19?
COVID-19 disease is caused by a coronavirus called SARS-CoV-2. You can get COVID-19 through contact with another person who has the virus. It is predominantly a respiratory illness that can affect other organs. People with COVID-19 have had a wide range of symptoms reported, ranging from mild symptoms to severe illness leading to death. Symptoms may appear 2 to 14 days after exposure to the virus. Symptoms may include: fever or chills; cough; shortness of breath; fatigue; muscle or body aches; headache; new loss of taste or smell; sore throat; congestion or runny nose; nausea or vomiting; diarrhea.
HOW ARE THE PFIZER-BIONTECH COVID-19 VACCINE AND THE PFIZER-BIONTECH COVID-19 VACCINE, BIVALENT RELATED?
The Pfizer-BioNTech COVID-19 Vaccine, Bivalent is made in the same way as the Pfizer-BioNTech COVID-19 Vaccine, but it also contains an Omicron component to help prevent COVID-19 caused by the Omicron variant of SARS-CoV-2.
For more information on EUA, see the "What is an Emergency Use Authorization (EUA)?" section at the end of this Fact Sheet.
WHAT SHOULD YOU MENTION TO YOUR CHILD'S VACCINATION PROVIDER BEFORE YOUR CHILD GETS ANY OF THESE VACCINES?
Tell the vaccination provider about all of your child's medical conditions, including if your child:
- •
- has any allergies
- •
- has had myocarditis (inflammation of the heart muscle) or pericarditis (inflammation of the lining outside the heart)
- •
- has a fever
- •
- has a bleeding disorder or is on a blood thinner
- •
- is immunocompromised or is on a medicine that affects your child's immune system
- •
- has received another COVID-19 vaccine
- •
- has ever fainted in association with an injection
HOW ARE THESE VACCINES GIVEN?
The Pfizer-BioNTech COVID-19 Vaccine or the Pfizer-BioNTech COVID-19 Vaccine, Bivalent, will be given to your child as an injection into the muscle.
Primary Series
The complete primary series consists of 3 doses administered over at least 11 weeks:
- •
- The Pfizer-BioNTech COVID-19 Vaccine is given for the initial 2 doses, which are administered 3 weeks apart.
- •
- The Pfizer-BioNTech COVID-19 Vaccine, Bivalent is given as the third dose, which is administered at least 8 weeks after the second dose.
Booster Dose
The Pfizer-BioNTech COVID-19 Vaccine, Bivalent is authorized for use in individuals 6 months through 4 years of age to provide a single booster dose given at least 2 months after completion of primary vaccination with 3 doses of the Pfizer-BioNTech COVID-19 Vaccine.
The vaccine may not protect everyone.
WHO SHOULD NOT GET THE PFIZER-BIONTECH COVID-19 VACCINE OR THE PFIZER-BIONTECH COVID-19 VACCINE, BIVALENT?
Your child should not get any of these vaccines if your child:
- •
- had a severe allergic reaction after a previous dose of the Pfizer-BioNTech COVID-19 Vaccine
- •
- had a severe allergic reaction to any ingredient in these vaccines.
WHAT ARE THE INGREDIENTS IN THESE VACCINES?
The Pfizer-BioNTech COVID-19 Vaccine and the Pfizer-BioNTech COVID-19 Vaccine, Bivalent include the following ingredients: mRNA, lipids (((4-hydroxybutyl)azanediyl)bis(hexane-6,1-diyl)bis(2-hexyldecanoate), 2 [(polyethylene glycol)-2000]-N,N-ditetradecylacetamide, 1,2-distearoyl-sn-glycero-3-phosphocholine, and cholesterol), tromethamine, tromethamine hydrochloride, sucrose, and sodium chloride.
HAVE THESE VACCINES BEEN USED BEFORE?
Millions of individuals 6 months of age and older have received the Pfizer-BioNTech COVID-19 Vaccine under EUA. In a clinical trial, approximately 1,200 individuals 6 months through 23 months of age, approximately 1,800 individuals 2 through 4 years of age, and approximately 3,100 individuals 5 through 11 years of age have received at least 1 dose of Pfizer-BioNTech COVID-19 Vaccine. In another clinical trial, approximately 23,000 individuals 12 years of age and older have received at least 1 dose of the Pfizer-BioNTech COVID-19 Vaccine.
Millions of individuals 5 years of age and older have received the Pfizer-BioNTech COVID-19 Vaccine, Bivalent under EUA. In clinical trials, 60 individuals 6 months through 4 years of age, 113 individuals 5 through 11 years of age, 107 individuals 12 through 17 years of age, 103 individuals 18 through 55 years of age, and 106 individuals greater than 55 years of age received a dose of Pfizer-BioNTech COVID-19 Vaccine, Bivalent. In a clinical trial, approximately 300 individuals greater than 55 years of age received 1 dose of a bivalent vaccine that differs from the Pfizer-BioNTech COVID-19 Vaccine, Bivalent in that it contains a different Omicron component.
WHAT ARE THE BENEFITS OF THESE VACCINES?
The Pfizer-BioNTech COVID-19 Vaccine has been shown to prevent COVID-19. FDA has authorized the Pfizer-BioNTech COVID-19 Vaccine, Bivalent to provide better protection against COVID-19 caused by the Omicron variant of SARS-CoV-2.
The duration of protection against COVID-19 is currently unknown.
WHAT ARE THE RISKS OF THESE VACCINES?
There is a remote chance that these vaccines could cause a severe allergic reaction. A severe allergic reaction would usually occur within a few minutes to one hour after getting a dose. For this reason, your child's vaccination provider may ask your child to stay at the place where your child received the vaccine for monitoring after vaccination. Signs of a severe allergic reaction can include:
- •
- Difficulty breathing
- •
- Swelling of the face and throat
- •
- A fast heartbeat
- •
- A bad rash all over the body
- •
- Dizziness and weakness
Myocarditis (inflammation of the heart muscle) and pericarditis (inflammation of the lining outside the heart) have occurred in some people who have received the Pfizer-BioNTech COVID-19 Vaccine and the Pfizer-BioNTech COVID-19 Vaccine, Bivalent. In most of these people, symptoms began within a few days following vaccination. The chance of having this occur is very low. You should seek medical attention right away if your child has any of the following symptoms after receiving the Pfizer-BioNTech COVID-19 Vaccine or the Pfizer-BioNTech COVID-19 Vaccine, Bivalent, particularly during the 2 weeks after your child receives a dose of either vaccine:
- •
- Chest pain
- •
- Shortness of breath or difficulty breathing
- •
- Feelings of having a fast-beating, fluttering, or pounding heart
- •
- Fainting
- •
- Unusual and persistent irritability
- •
- Unusual and persistent poor feeding
- •
- Unusual and persistent fatigue or lack of energy
- •
- Persistent vomiting
- •
- Persistent pain in the abdomen
- •
- Unusual and persistent cool, pale skin
Side effects that have been reported with these vaccines include:
- •
- Severe allergic reactions
- •
- Non-severe allergic reactions such as rash, itching, hives, or swelling of the face
- •
- Myocarditis (inflammation of the heart muscle)
- •
- Pericarditis (inflammation of the lining outside the heart)
- •
- Injection site pain/tenderness
- •
- Tiredness
- •
- Headache
- •
- Muscle pain
- •
- Chills
- •
- Joint pain
- •
- Fever
- •
- Injection site swelling
- •
- Injection site redness
- •
- Nausea
- •
- Feeling unwell
- •
- Swollen lymph nodes (lymphadenopathy)
- •
- Decreased appetite
- •
- Diarrhea
- •
- Vomiting
- •
- Arm pain
- •
- Fainting in association with injection of the vaccine
- •
- Dizziness
- •
- Irritability
These may not be all the possible side effects of these vaccines. Serious and unexpected side effects may occur. The possible side effects of these vaccines are still being studied.
WHAT SHOULD I DO ABOUT SIDE EFFECTS?
If your child experiences a severe allergic reaction, call 9-1-1, or go to the nearest hospital.
Call the vaccination provider or your child's healthcare provider if your child has any side effects that bother your child or do not go away.
Report vaccine side effects to FDA/CDC Vaccine Adverse Event Reporting System (VAERS). The VAERS toll-free number is 1-800-822-7967 or report online to https://vaers.hhs.gov/reportevent.html. Please include "Pfizer-BioNTech COVID-19 Vaccine EUA" or "Pfizer-BioNTech COVID-19 Vaccine, Bivalent EUA" in the first line of box #18 of the report form.
In addition, you can report side effects to Pfizer Inc. at the contact information provided below.
| Website | Fax number | Telephone number |
|---|---|---|
|
1-866-635-8337 |
1-800-438-1985 |
You may also be given an option to enroll in v-safe. V-safe is a voluntary smartphone-based tool that uses text messaging and web surveys to check in with people who have been vaccinated to identify potential side effects after COVID-19 vaccination. V-safe asks questions that help CDC monitor the safety of COVID-19 vaccines. V-safe also provides dose reminders if needed and live telephone follow-up by CDC if participants report a significant health impact following COVID-19 vaccination. For more information on how to sign up, visit: www.cdc.gov/vsafe.
WHAT IF I DECIDE NOT TO HAVE MY CHILD GET THE PFIZER-BIONTECH COVID-19 VACCINE OR THE PFIZER-BIONTECH COVID-19 VACCINE, BIVALENT?
Under the EUA, there is an option to accept or refuse receiving any of these vaccines. Should you decide for your child not to receive any of these vaccines, it will not change your child's standard medical care.
ARE OTHER CHOICES AVAILABLE FOR PREVENTING COVID-19 BESIDES PFIZER-BIONTECH COVID-19 VACCINE OR THE PFIZER-BIONTECH COVID-19 VACCINE, BIVALENT?
Other vaccines to prevent COVID-19 may be available under EUA.
CAN MY CHILD RECEIVE THE PFIZER-BIONTECH COVID-19 VACCINE OR THE PFIZER-BIONTECH COVID-19 VACCINE, BIVALENT AT THE SAME TIME AS OTHER VACCINES?
Data have not yet been submitted to FDA on administration of the Pfizer-BioNTech COVID-19 Vaccine or the Pfizer-BioNTech COVID-19 Vaccine, Bivalent at the same time with other vaccines. If you are considering having your child receive the Pfizer-BioNTech COVID-19 Vaccine or the Pfizer-BioNTech COVID-19 Vaccine, Bivalent with other vaccines, discuss the options with your child's healthcare provider.
WILL THESE VACCINES GIVE MY CHILD COVID-19?
No. These vaccines do not contain SARS-CoV-2 and cannot give your child COVID-19.
KEEP YOUR CHILD'S VACCINATION CARD
When your child gets the first COVID-19 vaccine, you will get a vaccination card. Remember to bring the card when your child returns.
ADDITIONAL INFORMATION
If you have questions, visit the website or call the telephone number provided below.
To access the most recent Fact Sheets, please scan the QR code provided below.
| Global website | Telephone number |
|---|---|
|
1-877-829-2619 |
HOW CAN I LEARN MORE?
- •
- Ask the vaccination provider.
- •
- Visit CDC at https://www.cdc.gov/coronavirus/2019-ncov/index.html.
- •
- Visit FDA at https://www.fda.gov/emergency-preparedness-and-response/mcm-legal-regulatory-and-policy-framework/emergency-use-authorization.
- •
- Contact your local or state public health department.
WHERE WILL MY CHILD'S VACCINATION INFORMATION BE RECORDED?
The vaccination provider may include your child's vaccination information in your state/local jurisdiction's Immunization Information System (IIS) or other designated system. For more information about IISs visit: https://www.cdc.gov/vaccines/programs/iis/about.html.
CAN I BE CHARGED AN ADMINISTRATION FEE FOR RECEIPT OF THESE COVID-19 VACCINES?
No. At this time, the provider cannot charge you for a vaccine dose and you cannot be charged an out-of-pocket vaccine administration fee or any other fee if only receiving a COVID-19 vaccination. However, vaccination providers may seek appropriate reimbursement from a program or plan that covers COVID-19 vaccine administration fees for the vaccine recipient (private insurance, Medicare, Medicaid, Health Resources & Services Administration [HRSA] COVID-19 Uninsured Program for non-insured recipients).
WHERE CAN I REPORT CASES OF SUSPECTED FRAUD?
Individuals becoming aware of any potential violations of the CDC COVID-19 Vaccination Program requirements are encouraged to report them to the Office of the Inspector General, U.S. Department of Health and Human Services, at 1-800-HHS-TIPS or https://TIPS.HHS.GOV.
WHAT IS THE COUNTERMEASURES INJURY COMPENSATION PROGRAM?
The Countermeasures Injury Compensation Program (CICP) is a federal program that may help pay for costs of medical care and other specific expenses of certain people who have been seriously injured by certain medicines or vaccines, including these vaccines. Generally, a claim must be submitted to the CICP within one (1) year from the date of receiving the vaccine. To learn more about this program, visit www.hrsa.gov/cicp/ or call 1-855-266-2427.
WHAT IS AN EMERGENCY USE AUTHORIZATION (EUA)?
An EUA is a mechanism to facilitate the availability and use of medical products, including vaccines, during public health emergencies, such as the current COVID-19 pandemic. An EUA is supported by a Secretary of Health and Human Services (HHS) declaration that circumstances exist to justify the emergency use of drugs and biological products during the COVID-19 pandemic. A product authorized for emergency use has not undergone the same type of review by FDA as an FDA-approved product.
FDA may issue an EUA when certain criteria are met, which includes that there are no adequate, approved, and available alternatives. In addition, the FDA decision is based on the totality of the scientific evidence available showing that the product may be effective to prevent COVID-19 during the COVID-19 pandemic and that the known and potential benefits of the product outweigh the known and potential risks of the product. All of these criteria must be met to allow for the product to be used during the COVID-19 pandemic.
An EUA is in effect for the duration of the COVID-19 EUA declaration justifying emergency use of this product, unless terminated or revoked (after which the product may no longer be used).

Manufactured for
BioNTech Manufacturing GmbH
An der Goldgrube 12
55131 Mainz, Germany

Manufactured by
Pfizer Inc., New York, NY 10001
LAB-1517-5.0
Revised: 14 March 2023
 |
Scan to capture that this Fact Sheet was provided to vaccine recipient for the electronic medical records/immunization information systems. |
|
GDTI: 0886983000486 |
|
- 6
- The Pfizer-BioNTech COVID-19 Vaccine, a monovalent vaccine, encodes the spike protein of only the Original SARS-CoV-2.
- 7
- The Pfizer-BioNTech COVID-19 Vaccine, Bivalent encodes the spike protein of the Original SARS-CoV-2 and the Omicron BA.4/BA.5 SARS-CoV-2.
- 8
- If your child will turn 5 years of age in the next 11 weeks and has not started their primary series, your child may receive either: (1) a 3-dose primary series as described in this Fact Sheet; or (2) a 2-dose primary series with the Pfizer-BioNTech COVID-19 Vaccine authorized for use in individuals 5 years through 11 years of age. Please discuss the options with your provider.
- 9
- You may receive this Fact Sheet if your child is 5 years old and previously received a dose of the Pfizer-BioNTech COVID-19 Vaccine authorized for use in individuals 6 months through 4 years of age. Your child may complete the 3-dose primary series as described in this Fact Sheet.
- 10
- The Pfizer-BioNTech COVID-19 Vaccine is no longer authorized for use as the third dose of the primary series in individuals 6 months through 4 years of age.
PRINCIPAL DISPLAY PANEL - 2.6 mL Vial Label
Pfizer-BioNTech COVID-19 Vaccine
DILUTE PRIOR TO USE
Age 6m - 4y
After dilution -10 doses of 0.2 mL
For intramuscular use. Contains no preservative.
For use under Emergency Use Authorization.
After dilution store at 2 to 25°C (35 to 77°F) and
discard after 12 hours.
Dilution date and time:
NDC 59267-0078-1
| PFIZER-BIONTECH COVID-19 VACCINE
bnt162b2 injection, suspension |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - Pfizer Manufacturing Belgium NV (370156507) |
| Registrant - Pfizer Inc (113480771) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Pfizer Manufacturing Belgium NV | 370156507 | ANALYSIS(59267-0078) , MANUFACTURE(59267-0078) , PACK(59267-0078) , LABEL(59267-0078) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Wyeth BioPharma Division of Wyeth Pharmaceuticals LLC | 174350868 | ANALYSIS(59267-0078) , API MANUFACTURE(59267-0078) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Pfizer Inc | 004954111 | ANALYSIS(59267-0078) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Pfizer Ireland Pharmaceuticals | 985586408 | ANALYSIS(59267-0078) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| BioNTech Innovative Manufacturing Services GmbH | 537365801 | ANALYSIS(59267-0078) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| BioNTech Manufacturing GmbH | 314382536 | ANALYSIS(59267-0078) , API MANUFACTURE(59267-0078) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| BioNTech Manufacturing Marburg GmbH | 313270335 | ANALYSIS(59267-0078) , API MANUFACTURE(59267-0078) | |