Label: ERGOTAMINE- ergotamine tartrate and caffeine tablet, film coated
-
Contains inactivated NDC Code(s)
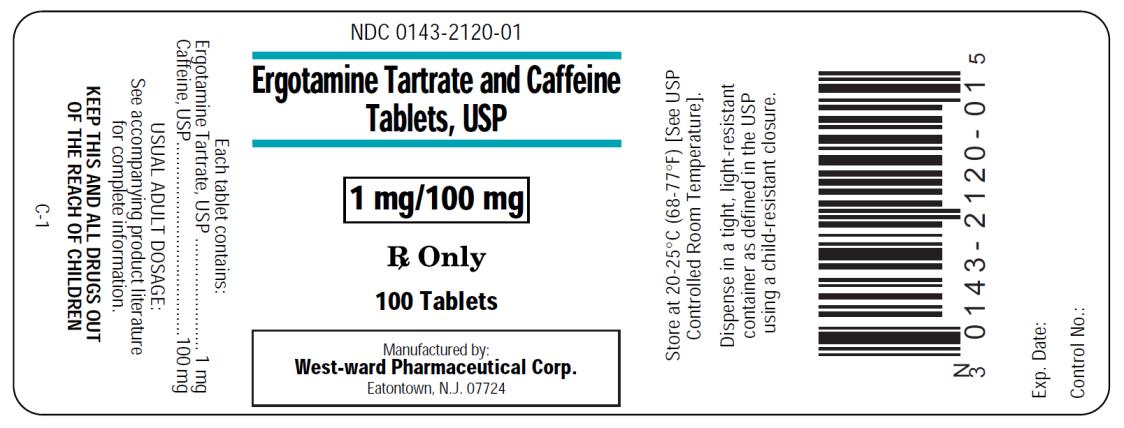
NDC Code(s): 0143-2120-01, 0143-2120-05, 0143-2120-30 - Packager: West-Ward Pharmaceutical Corp
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated April 10, 2013
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
BOXED WARNING
(What is this?)
WARNING
Serious and/or life-threatening peripheral ischemia has been associated with the coadministration of ergotamine tartrate and caffeine with potent CYP 3A4 inhibitors including protease inhibitors and macrolide antibiotics. Because CYP 3A4 inhibition elevates the serum levels of ergotamine tartrate and caffeine, the risk for vasospasm leading to cerebral ischemia and/or ischemia of the extremities is increased. Hence, concomitant use of these medications is contraindicated. (See also CONTRAINDICATIONS and WARNINGS sections)
-
DESCRIPTION:
Ergotamine Tartrate and Caffeine Tablets USP
ergotamine tartrate USP . . . . . . . . . . . . . . . . . .1 mg
caffeine USP . . . . . . . . . . . . . . . . . . . . . . . . . . .100 mg
In addition, each tablet contains the following inactive ingredients: colloidal silicon dioxide, compressible sugar, corn starch, magnesium stearate, mannitol, microcrystalline cellulose, sodium starch glycolate, sugar, and tartaric acid. Polishing and Film Coating Solutions may contain the following: FD&C Blue Lake #2, FD&C Yellow Lake #6, hydroxypropyl methylcellulose, polyethylene glycol, and titanium dioxide. The printing ink contains: amide resin, black pigment, natural resin, and wax.
-
CLINICAL PHARMACOLOGY
Ergotamine is an alpha adrenergic blocking agent with a direct stimulating effect on the smooth muscle of peripheral and cranial blood vessels and produces depression of central vasomotor centers. The compound also has the properties of serotonin antagonism. In comparison to hydrogenated ergotamine, the adrenergic blocking actions are less pronounced and vasoconstrictive actions are greater.
Caffeine, also a cranial vasoconstrictor, is added to further enhance the vasoconstrictive effect without the necessity of increasing ergotamine dosage.
Many migraine patients experience excessive nausea and vomiting during attacks, making it impossible for them to retain any oral medication. In such cases, therefore, the only practical means of medication is through the rectal route where medication may reach the cranial vessels directly, evading the splanchnic vasculature and the liver.
Pharmacokinetics: Interactions
Pharmacokinetic interactions (increased blood levels of ergotamine) have been reported in patients treated orally with ergotamine and macrolide antibiotics (e.g., troleandomycin, clarithromycin, erythromycin), and in patients treated orally with ergotamine and protease inhibitors (e.g. ritonavir) presumably due to inhibition of cytochrome P450 3A metabolism of ergotamine (see CONTRAINDICATIONS). Ergotamine has also been shown to be an inhibitor of cytochrome P450 3A catalyzed reactions. No pharmacokinetic interactions involving other cytochrome P450 isoenzymes are known.
- INDICATIONS AND USAGE
-
CONTRAINDICATIONS
Coadministration of ergotamine with potent CYP 3A4 inhibitors (ritonavir, nelfinavir, indinavir, erythromycin, clarithromycin, and troleandomycin) has been associated with acute ergot toxicity (ergotism) characterized by vasospasm and ischemia of the extremities (see PRECAUTIONS: Drug Interactions), with some cases resulting in amputation. There have been rare reports of cerebral ischemia in patients on protease inhibitor therapy when ergotamine tartrate and caffeine was coadministered, at least one resulting in death. Because of the increased risk for ergotism and other serious vasospastic adverse events, ergotamine use is contraindicated with these drug and other potent inhibitors of CYP 3A4 (e.g., ketoconazole, itraconazole) (see WARNINGS: CYP 3A4 Inhibitors).
Ergotamine tartrate and caffeine may cause fetal harm when administered to pregnant women. Ergotamine tartrate and caffeine is contraindicated in women who are or may become pregnant. If this drug is used during pregnancy or if the patient becomes pregnant while taking this product, the patient should be apprised of the potential hazard to the fetus.
Peripheral vascular disease, coronary heart disease, hypertension, impaired hepatic or renal function and sepsis.
Hypersensitivity to any of the components.
-
WARNINGS
CYP 3A4 Inhibitors (e.g. Macrolide Antibiotics and Protease Inhibitors)
Coadministration of ergotamine with potent CYP 3A4 inhibitors such as protease inhibitors or macrolide antibiotics has been associated with serious adverse events, for this reason, these drugs should not be given concomitantly with ergotamine (see CONTRAINDICATIONS). While these reactions have not been reported with less potent CYP 3A4 inhibitors, there is a potential risk for serious toxicity including vasospasm when these drugs are used with ergotamine. Examples of less potent CYP 3A4 inhibitors include: saquinavir, nefazodone, fluconazole, fluoxetine, grapefruit juice, fluvoxamine, zileuton, metronidazole, and clotrimazole. These lists are not exhaustive, and the prescriber should consider the effects on CYP3A4 of other agents being considered for concomitant use with ergotamine.
Fibrotic Complications
There have been a few reports of patients on ergotamine tartrate and caffeine therapy developing retroperitoneal and/or pleuropulmonary fibrosis. There have also been rare reports of fibrotic thickening of the aortic, mitral, tricuspid, and/or pulmonary valves with long-term continuous use of ergotamine tartrate and caffeine. Ergotamine tartrate should not be used for chronic daily administration (see DOSAGE AND ADMINISTRATION).
-
PRECAUTIONS
General
Although signs and symptoms of ergotism rarely develop even after long term intermittent use of the rectally administered drug, care should be exercised to remain within the limits of recommended dosage.
Ergotism is manifested by intense arterial vasoconstriction, producing signs and symptoms of peripheral vascular ischemia. Ergotamine induces vasoconstriction by a direct action on vascular smooth muscle. In chronic intoxication with ergot derivatives, headache, intermittent claudication, muscle pains, numbness, coldness and pallor of the digits may occur. If the condition is allowed to progress untreated, gangrene can result.
While most cases of ergotism associated with ergotamine treatment result from frank overdosage, some cases have involved apparent hypersensitivity. There are few reports of ergotism among patients taking doses within the recommended limits or for brief periods of time. In rare instances, patients, particularly those who have used the medication indiscriminately over long periods of time, may display withdrawal symptoms consisting of rebound headache upon discontinuation of the drug.
Rare cases of solitary rectal or anal ulcer have occurred from abuse of ergotamine suppositories usually in higher than recommended doses or with continual use at the recommended dose for many years. Spontaneous healing occurs within usually 4-8 weeks after drug withdrawal.
Information for Patients
Patients should be advised that two tablets of ergotamine tartrate and caffeine should be taken at the first sign of a migraine headache. No more than 6 tablets should be taken for any single migraine attack. No more than 10 tablets should be taken during any 7-day period. Administration of ergotamine tartrate and caffeine tablets should not exceed the dosing guidelines and should be used for chronic daily administration (see DOSAGE AND ADMINISTRATION). Ergotamine tartrate and caffeine should be used only for migraine headaches. It is not effective for other types of headaches and it lacks analgesic properties. Patients should be advised to report to the physician immediately any of the following: numbness or tingling in the fingers and toes, muscle pain in the arms and legs, weakness in the legs, pain in the chest or temporary speeding or slowing of the heart rate, swelling or itching.
Drug Interactions
CYP 3A4 Inhibitors (e.g. Macrolide Antibiotics and Protease Inhibitors)
See CONTRAINDICATIONS and WARNINGS.
Ergotamine tartrate and caffeine should not be administered with other vasoconstrictors. Use with sympathominetics (pressor agents) may cause extreme elevation of blood pressure. The beta-blocker Inderal (propranolol) has been reported to potentiate the vasoconstrictive action of ergotamine tartrate and caffeine by blocking the vasodilating property of epinephrine. Nicotine may provoke vasoconstriction in some patients, predisposing to a greater ischemic response to ergot therapy.
The blood levels of ergotamine-containing drugs are reported to be elevated by the concomitant administration of macrolide antibiotics and vasospastic reactions have been reported with therapeutic doses of the ergotamine-containing drugs when coadministered with those antibiotics.
Teratogenic Effects
Pregnancy Category X: There are no studies on the placental transfer or teratogenicity of the combined products of ergotamine tartrate and caffeine. Caffeine is known to cross the placenta and has been shown to be teratogenic in animals. Ergotamine crosses the placenta in small amounts, although it does not appear to be embryotoxic in this quantity. However, prolonged vasoconstriction of the uterine vessels and/or increased myometrial tone leading to reduced myometrial and placental blood flow may have contributed to fetal growth retardation observed in animals (see CONTRAINDICATIONS).
Nonteratogenic Effects
Ergotamine tartrate and caffeine is contraindicated in pregnancy due to the oxytocic effects of ergotamine (see CONTRAINDICATIONS).
Labor and Delivery
Ergotamine tartrate and caffeine is contraindicated in labor and delivery due to its oxytocic effect which is maximal in the third trimester (see CONTRAINDICATIONS).
Nursing Mothers
Ergot drugs are known to inhibit prolactin but there are no reports of decreased lactation with ergotamine tartrate and caffeine. Ergotamine is excreted in breast milk and may cause symptoms of vomiting, diarrhea, weak pulse and unstable blood pressure in nursing infants. Because of the potential for serious adverse reactions in nursing infants from ergotamine tartrate and caffeine, a decision should be made whether to discontinue nursing or discontinue the drug, taking into account the importance of the drug to the mother.
-
ADVERSE REACTIONS:
Cardiovascular: Vasoconstrictive complications of a serious nature may occur at times. These include ischemia, cyanosis, absence of pulse, cold extremities, gangrene, precordial distress and pain, EKG changes and muscle pains. Although these effects occur most commonly with long-term therapy at relatively high doses, they have also been reported with short-term or normal doses. Other cardiovascular adverse effects include transient tachycardia or bradycardia and hypertension.
Gastrointestinal: Nausea and vomiting; rectal or anal ulcer (from overuse of suppositories).
Neurological: paresthesias, numbness, weakness, and vertigo.
Allergic: Localized edema and itching.
Fibrotic Complications: (see WARNINGS).
-
DRUG ABUSE AND DEPENDENCE
There have been reports of drug abuse and psychological dependence in patients on ergotamine tartrate and caffeine therapy. Due to chronicity of vascular headaches, it is imperative that patients be advised not to exceed recommended dosages with long-term use to avoid ergotism (see PRECAUTIONS).
-
OVERDOSAGE
The toxic effects of an acute overdosage of ergotamine tartrate and caffeine are due primarily to the ergotamine component. The amount of caffeine is such that its toxic effects will be overshadowed by those of ergotamine. Symptoms include vomiting, numbness, tingling, pain and cyanosis of the extremities associated with diminished or absent peripheral pulses, hypertension or hypotension, drowsiness, stupor, coma, convulsion and shock. A case has been reported of reversible bilateral papillitis with ring scotomata in a patient who received five times the recommended daily adult dose over a period of 14 days.
Treatment consists of removal of the offending drug by induction of emesis. Maintenance of adequate pulmonary ventilation, correction of hypotension, and control of convulsions and blood pressure are important considerations. Treatment of peripheral vasospasm should consist of warmth, but not heat, and protection of the ischemic limbs. Vasodilators may be beneficial but caution must be exercised to avoid aggravating an already existent hypotension.
-
DOSAGE AND ADMINISTRATION
Procedure: For the best results, dosage should start at the first sign of an attack.
Adults: Take 2 tablets at the start of attack; 1 additional tablet every ½ hour, if needed for full relief (maximum 6 tablets per attack, 10 per week).
Early Administration Gives Maximum Effectiveness.
Maximum Adult Dosage: Six tablets is the maximum dose for an individual attack.
Total weekly dosage should not exceed 10 tablets. Ergotamine tartrate and caffeine-tablets should not be used for chronic daily administration.
In carefully selected patients, with due consideration of maximum dosage recommendations, administration of the drug at bedtime may be an appropriate short-term preventive measure.
-
HOW SUPPLIED
Ergotamine Tartrate and Caffeine Tablets USP, 1 mg/100 mg are round, film coated buff colored tablet; printed "WW 120" in black ink and are available in:
Bottles of 30 tablets.
Bottles of 100 tablets.
Bottles of 500 tablets.
Store at 20°-25°C (68°-77°F) [See USP Controlled Room Temperature].
Dispense in a tight, light-resistant container as defined in the USP using a child-resistant closure.
Manufactured By:
West-ward Pharmaceutical Corp.
Eatontown, NJ 07724
Revised November 2004 - PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ERGOTAMINE
ergotamine tartrate and caffeine tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0143-2120 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ERGOTAMINE TARTRATE (UNII: MRU5XH3B48) (ERGOTAMINE - UNII:PR834Q503T) ERGOTAMINE TARTRATE 1 mg CAFFEINE (UNII: 3G6A5W338E) (CAFFEINE - UNII:3G6A5W338E) CAFFEINE 100 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) STARCH, CORN (UNII: O8232NY3SJ) MAGNESIUM STEARATE (UNII: 70097M6I30) MANNITOL (UNII: 3OWL53L36A) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) SUCROSE (UNII: C151H8M554) TARTARIC ACID (UNII: W4888I119H) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) HYPROMELLOSES (UNII: 3NXW29V3WO) POLYETHYLENE GLYCOLS (UNII: 3WJQ0SDW1A) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Product Characteristics Color WHITE Score no score Shape ROUND Size 10mm Flavor Imprint Code WW;120 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0143-2120-01 100 in 1 BOTTLE, PLASTIC 2 NDC:0143-2120-05 500 in 1 BOTTLE, PLASTIC 3 NDC:0143-2120-30 30 in 1 BOTTLE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA040510 09/17/2004 Labeler - West-Ward Pharmaceutical Corp (001230762)