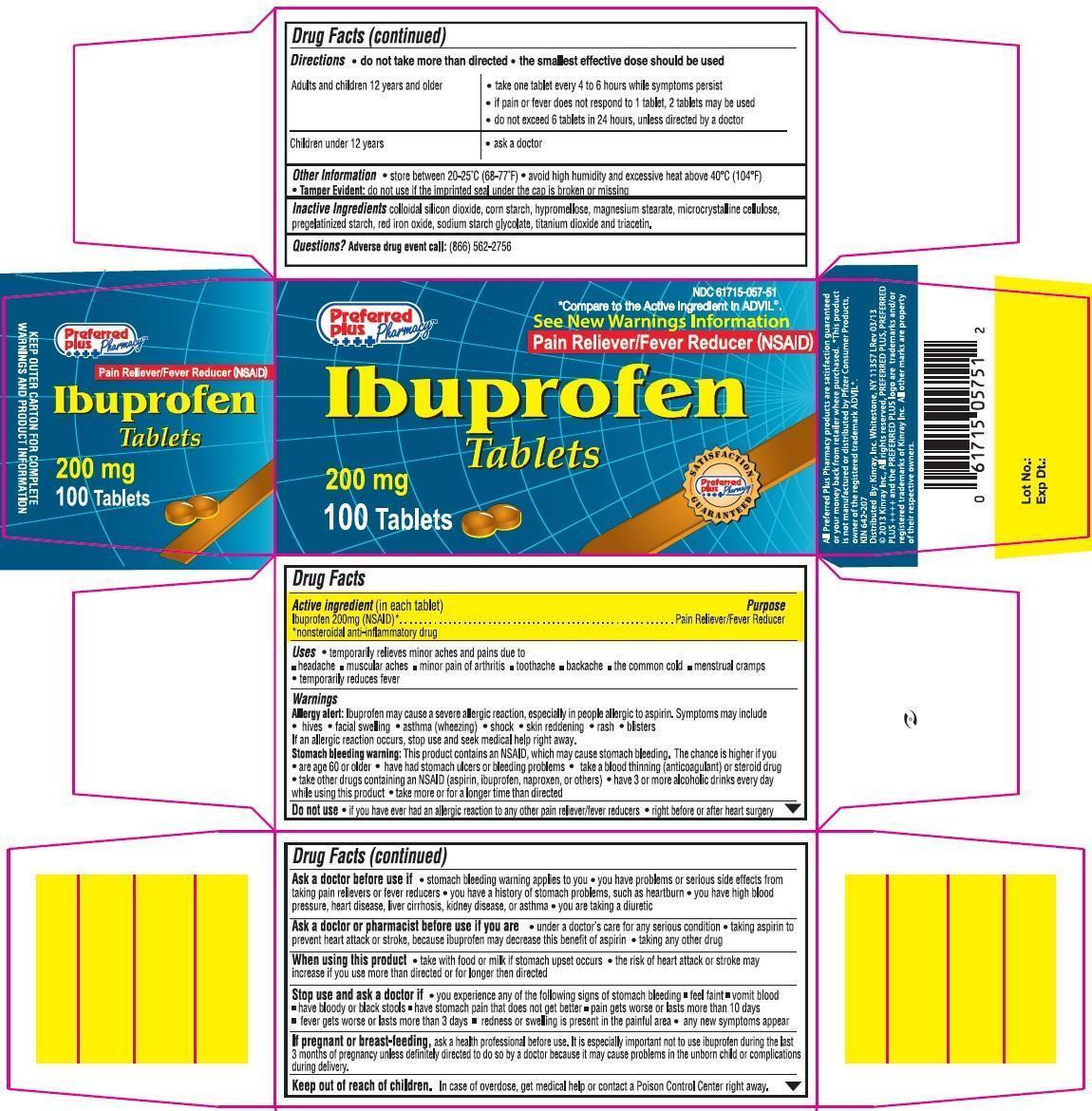
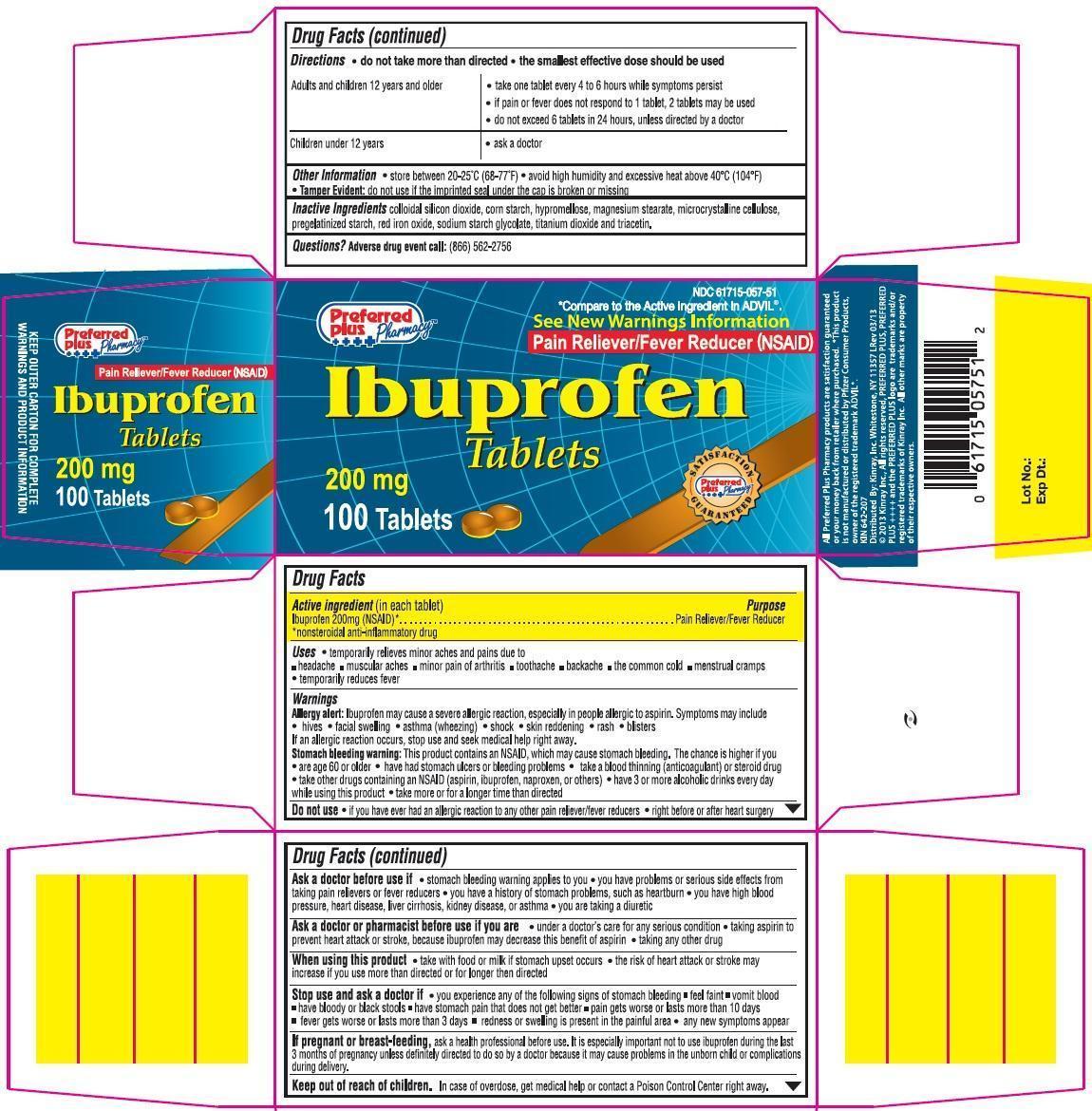
Label: PREFERRED PLUS IBUPROFEN- ibuprofen tablet, film coated
-
Contains inactivated NDC Code(s)
NDC Code(s): 61715-057-50, 61715-057-51 - Packager: Kinray, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated May 11, 2013
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient (in each tablet)
- Purpose
- Uses
-
Warnings
Allergy alert: Ibuprofen may cause a severe allergic reaction, especially in people allergic to aspirin. Symptoms may include
- hives
- facial swelling
- asthma (wheezing)
- shock
- skin reddening
- rash
- blisters
If an allergic reaction occurs, stop use and seek medical help right away.
Stomach bleeding warning: This product contains an NSAID, which may cause severe stomach bleeding. The chance is higher if you
- are age 60 or older
- have had stomach ulcers or bleeding problems
- take a blood thinning (anticoagulant) or steroid drug
- take other drugs containing an NSAID [aspirin, ibuprofen, naproxen, or others]
- have 3 or more alcoholic drinks everyday while using this product
- take more or for a longer time than directed
Do not use
- if you have ever had an allergic reaction to any other pain reliever/ fever reducer
- right before or after heart surgery
Ask a doctor before use if
- stomach bleeding warning applies to you
- you have problems or serious side effects from taking pain relievers or fever reducers
- you have a history of stomach problems, such as heartburn
- you have high blood pressure, heart disease, liver cirrhosis, kidney disease or asthma
- you are taking a diuretic
Ask a doctor or pharmacist before use if you are
- under a doctor's care for any serious condition
- taking aspirin to prevent heart attack or stroke, because ibuprofen may decrease this benefit of aspirin
- taking any other drug
when using this product
- take food or milk if stomach upset occurs
- the risk of heart attack or stroke may increase if you use more than directed or for longer than directed
stop use and ask a doctor if
you experience any of the following signs of stomach bleeding
- feel faint
- vomit blood
- have bloody or black stools
- have stomach pain that does not get better
- pain gets worse or last for more than 10 days
- fever gets worse or last more than 3 days
- redness or swelling is present in the painful area
-
Directions
-
do not take more than directed
- the smallest effective dose should be used
Adults and children 12 years and older
- take one tablet every 4 to 6 hours while symptoms persist
- if pain or fever does not respond to 1 tablet, 2 tablets may be used
- do not exceed 6 tablets in 24 hours, unless directed by a doctor
children under 12 years
- ask a doctor
-
do not take more than directed
- other information
- Inactive ingredients
- Questions?
-
Principal Display Panel
Ibuprofen Tablets 200 mg
*Compare to the active ingredient in ADVIL®.
See New Warnings Information
Pain Reliever/Fever Reducer (NSAID)
KEEP OUTER CARTON FOR COMPLETE WARNING AND PRODUCT INFORMATION
All Preferred Plus Pharmacy products are satisfaction guaranteed or your money back from retailer where purchased.
*This product is not manufactured or distributed by Pfizer Consumer Products, owner of the registered trademark ADVIL®.
Distributed By: Kinray, Inc.
Whitestone, NY 11357
©2013 Kinray, Inc.,All rights reserved, PREFERRED PLUS, PREFERRED PLUS ++++ and the PREFERRED PLUS logo are trademarks of Kinray, Inc. All other marks are property of their respective owners.
Product Label
-
INGREDIENTS AND APPEARANCE
PREFERRED PLUS IBUPROFEN
ibuprofen tablet, film coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:61715-057 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength IBUPROFEN (UNII: WK2XYI10QM) (IBUPROFEN - UNII:WK2XYI10QM) IBUPROFEN 200 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) STARCH, CORN (UNII: O8232NY3SJ) SODIUM STARCH GLYCOLATE TYPE A CORN (UNII: AG9B65PV6B) TRIACETIN (UNII: XHX3C3X673) HYPROMELLOSES (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) FERRIC OXIDE RED (UNII: 1K09F3G675) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color brown Score no score Shape ROUND Size 10mm Flavor Imprint Code IBU200 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:61715-057-50 1 in 1 CARTON 1 50 in 1 BOTTLE, PLASTIC 2 NDC:61715-057-51 1 in 1 CARTON 2 100 in 1 BOTTLE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA079129 05/11/2013 Labeler - Kinray, Inc. (012574513) Registrant - Pharbest Pharmaceuticals, Inc. (557054835) Establishment Name Address ID/FEI Business Operations Pharbest Pharmaceuticals, Inc. 557054835 repack(61715-057) , relabel(61715-057)