Label: TRIPHROCAPS- folic acid, ascorbic acid, niacinamide, thiamine mononitrate, riboflavin, pyridoxine hydrochloride, cyanocobalamin, calcium pantothenate, and biotin capsule
- NHRIC Code(s): 13811-525-01
- Packager: Trigen Laboratories, LLC
- Category: DIETARY SUPPLEMENT
Drug Label Information
Updated November 24, 2021
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SUPPLEMENT FACTS
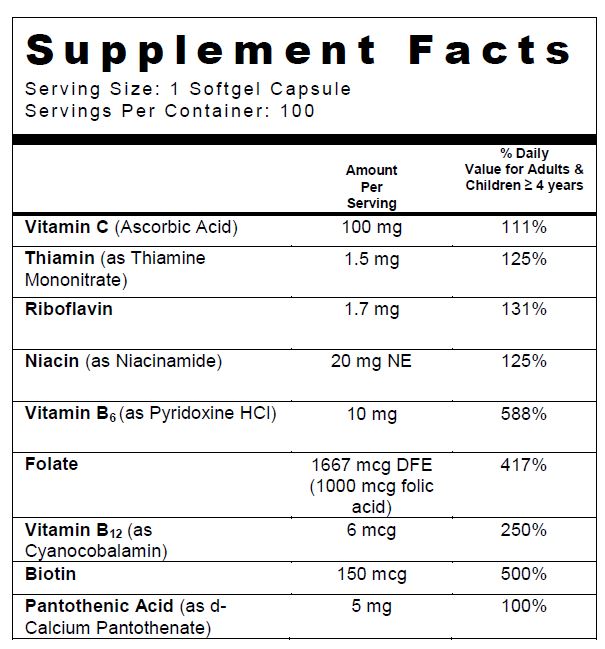
Other Ingredients: Soybean Oil, Bovine Gelatin, Glycerin, Water, Yellow Beeswax, Lecithin, Titanium Dioxide, FD&C Red #40, Ethyl Vanillin, FD&C Yellow #5, FD&C Yellow #6, and Iron Oxide Red
THIS PRODUCT CONTAINS SOY.
TriphroCaps are an orally administered prescription vitamin used for the dietary management of patients with nutritional deficiencies or are in need of nutritional supplementation.
- CONTRAINDICATIONS
-
PRECAUTIONS
Folic acid alone is an improper therapy in the treatment of pernicious anemia and other megaloblastic anemias where Vitamin B12 is deficient. Folic acid in doses above 0.1 mg daily may obscure pernicious anemia in that hematologic remission can occur while neurological manifestations remain progressive.
The patient’s medical conditions and consumption of other drugs, herbs, and/or supplements should be considered.
Pediatric Use: Safety and effectiveness in pediatric patients have not been established.
Geriatric Use: Safety and effectiveness in elderly patients have not been established.
Biotin levels higher than the recommended daily allowance may cause interference with some laboratory tests, including cardiovascular diagnostic tests (e.g. troponin) and hormone tests, and may lead to incorrect test results. Tell your healthcare provider about all prescription and over-the-counter medicines, vitamins, and dietary supplements that you take, including biotin.
This product contains FD&C Yellow No. 5 (tartrazine) which may cause allergic-type reactions (including bronchial asthma) in certain susceptible patients. Although the overall incidence of FD&C Yellow No. 5 (tartrazine) sensitivity in the general population is low, it is frequently seen in patients who also have aspirin hypersensitivity.
- DRUG INTERACTIONS
- ADVERSE REACTIONS
- DESCRIPTION
- DIRECTIONS FOR USE
- HOW SUPPLIED
- STORAGE
-
HEALTH CLAIM
KEEP OUT OF REACH OF CHILDREN.
For use on the order of a healthcare practitioner.
Call your doctor about side effects. To report side effects, call Trigen Laboratories, LLC at 1-770-509-4500 or FDA at 1-800-FDA-1088.
PLR-TRIPHRO-00001-1 Rev. 11/2021
Manufactured for:
Trigen Laboratories, LLC
Alpharetta, GA 30005

- PRINCIPAL DISPLAY PANEL - 100 Softgel Bottle Label
-
INGREDIENTS AND APPEARANCE
TRIPHROCAPS
folic acid, ascorbic acid, niacinamide, thiamine mononitrate, riboflavin, pyridoxine hydrochloride, cyanocobalamin, calcium pantothenate, and biotin capsuleProduct Information Product Type DIETARY SUPPLEMENT Item Code (Source) NHRIC:13811-525 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength folic acid (UNII: 935E97BOY8) (folic acid - UNII:935E97BOY8) folic acid 1 mg ascorbic acid (UNII: PQ6CK8PD0R) (ascorbic acid - UNII:PQ6CK8PD0R) ascorbic acid 100 mg niacinamide (UNII: 25X51I8RD4) (niacinamide - UNII:25X51I8RD4) niacinamide 20 mg thiamine mononitrate (UNII: 8K0I04919X) (THIAMINE ION - UNII:4ABT0J945J) Thiamine 1.5 mg riboflavin (UNII: TLM2976OFR) (riboflavin - UNII:TLM2976OFR) riboflavin 1.7 mg pyridoxine Hydrochloride (UNII: 68Y4CF58BV) (PYRIDOXINE - UNII:KV2JZ1BI6Z) pyridoxine Hydrochloride 10 mg cyanocobalamin (UNII: P6YC3EG204) (cyanocobalamin - UNII:P6YC3EG204) cyanocobalamin 6 ug calcium pantothenate (UNII: 568ET80C3D) (PANTOTHENIC ACID - UNII:19F5HK2737, CALCIUM CATION - UNII:2M83C4R6ZB) PANTOTHENIC ACID 5 mg biotin (UNII: 6SO6U10H04) (biotin - UNII:6SO6U10H04) biotin 150 ug Inactive Ingredients Ingredient Name Strength soybean oil (UNII: 241ATL177A) GELATIN TYPE B BOVINE (160 BLOOM) (UNII: 1T8387508X) glycerin (UNII: PDC6A3C0OX) water (UNII: 059QF0KO0R) yellow wax (UNII: 2ZA36H0S2V) titanium dioxide (UNII: 15FIX9V2JP) FD&C Red No. 40 (UNII: WZB9127XOA) ethyl vanillin (UNII: YC9ST449YJ) FD&C yellow No. 5 (UNII: I753WB2F1M) FD&C yellow No. 6 (UNII: H77VEI93A8) ferric oxide red (UNII: 1K09F3G675) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:13811-525-01 100 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date DIETARY SUPPLEMENT 01/27/2016 Supplement Facts Serving Size : Serving per Container : Amount Per Serving % Daily Value color imprint scoring 1 shape size (solid drugs) 13 mm Labeler - Trigen Laboratories, LLC (830479668)


