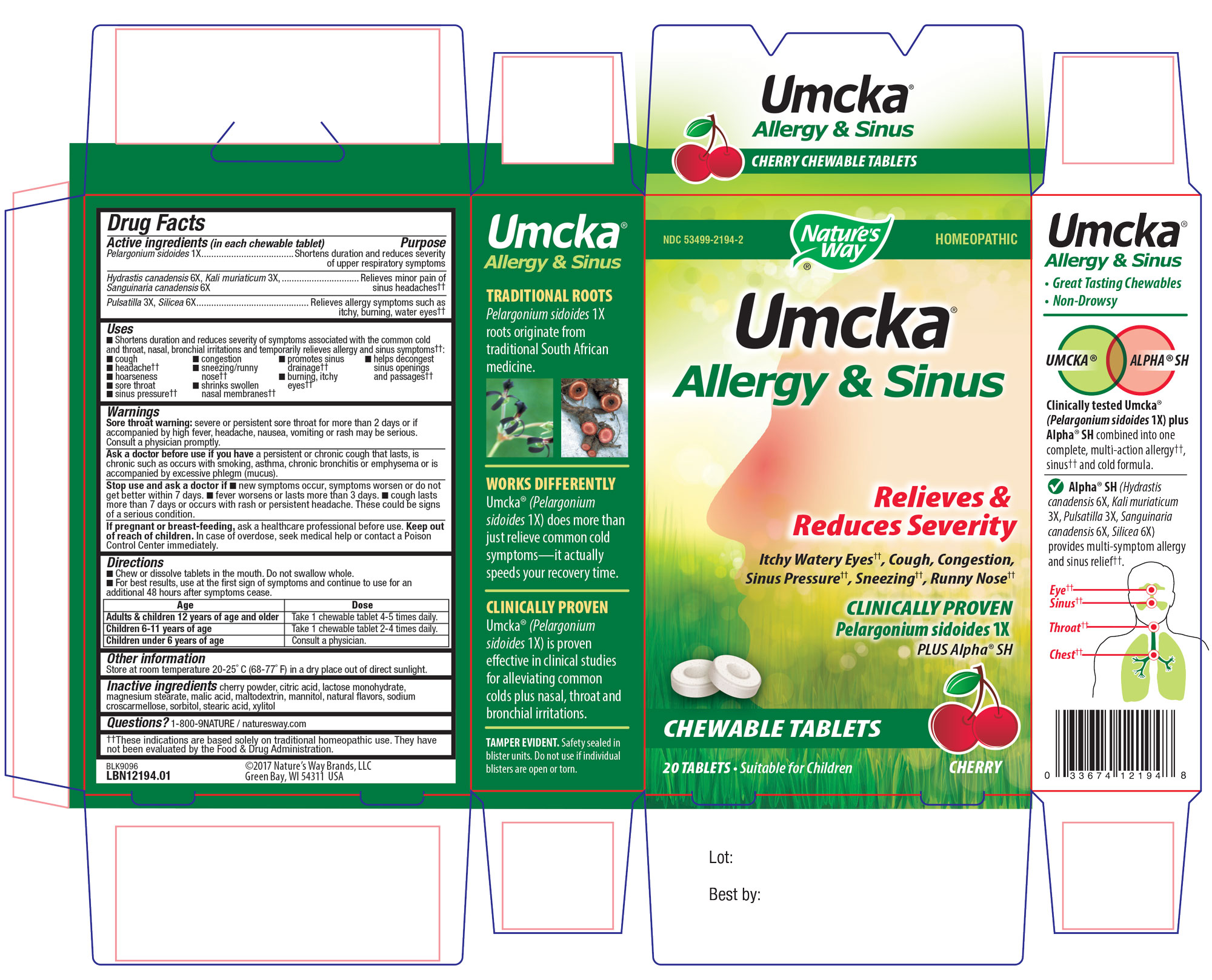
UMCKA ALLERGY AND SINUS- pelargonium sidoides root, hydrastis canadensis whole, potassium chloride, sanguinaria canadensis root, pulsatilla vulgaris, silicon dioxide tablet, chewable
Schwabe North American, Inc.
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
Umcka Allergy and Sinus
Active Ingredient Section
Pelargonium sidoides 1X,
Hydrastis canadensis 6X,
Kali muriaticum 3X (Potassium chloride),
Sanguinaria canadensis 6X,
Pulsatilla 3X,
Silicea 6X (Silicon Dioxide)
Inactive Ingredient Section
Cherry Powder,
Citric Acid,
Lactose monohydrate,
Magnesium Sterate,
Malic Acid,
Maltodextrin,
Mannitol,
Natural Flavors,
Croscarmellose Sodium,
Sorbitol,
Stearic acid,
Xylitol
Purpose Section
Shortens duration and reduces severity of symptoms associated with the common cold and throat, nasal, bronchial irritations and temporarily relieves allergy and sinus symptoms; cough, headache, hoarseness, sore throat, sinus pressure, congestion, sneezing/runny nose, shrinks swollen nasal membrances, promotes sinus drainage, burning, itchy eyes, helps decongest sinus openings and passages
Indications and Usage Section
Shortens duration and reduces severity of symptoms associated with the common cold and throat, nasal, bronchial irritations and temporarily relieves allergy and sinus symptoms; cough, headache, hoarseness, sore throat, sinus pressure, congestion, sneezing/runny nose, shrinks swollen nasal membrances, promotes sinus drainage, burning, itchy eyes, helps decongest sinus openings and passages
Warnings Section
Sore throat warning: severe or persistent sore throat for more than 2 days or if accompanied by high fever, headache, nausea, vomiting or rash maybe serious.
Consult a physician promptly.
Ask a Doctor Section
Ask a doctor before use if you have a persistant or chronic cough that lasts, is chronic such as ocurs with smoking, asthma, chronic bronchitis, or emphysema or is accompanied by excessive phlegm (mucus).
Stop Use Section
Stop use and ask a doctor is new symptoms occur, symptoms worsen or do not get better within 7 days, fever worsens or lasts more than 3 days, cough lasts more than 7 days or occurs with rash or persistent headache.
These could be signs of a serious condition.
Pregnancy or Breast Feeding Section
If pregnant or breast-feeding, ask a healthcare professional before use.
Keep Out of Reach of Children Section
Keep out of reach of children. In case of overdose, seek medical help or contact a Poison Control Center immediatley.
Dosage and Administration Section
Chew or dissolve tablets in the mouth.
Do not swallow whole.
For best resutls, use at the first sign of symptoms and continue to use for an additional 48 hours after symptoms cease.
Adults and children 12 years of age and older: take 1 chewable tablet 4-5 times daily.
Children 6-11 years of age: Take 1 chewable tablet 2-4 times daily.
Children under 6 years of age: Consult a physician.
| UMCKA ALLERGY AND SINUS
pelargonium sidoides root, hydrastis canadensis whole, potassium chloride, sanguinaria canadensis root, pulsatilla vulgaris, silicon dioxide tablet, chewable |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| Labeler - Schwabe North American, Inc. (831153908) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Schwabe North America, Inc. | 831153908 | manufacture(53499-2194) | |