Label: ALOQUIN- aloe vera leaf and iodoquinol gel
-
Contains inactivated NDC Code(s)
NDC Code(s): 68040-706-01, 68040-706-08, 68040-706-16 - Packager: Primus Pharmaceuticals
- Category: HUMAN PRESCRIPTION DRUG LABEL
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated October 7, 2009
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
Each gram of ALOQUIN contains 1.25% (12.5 mg) Iodoquinol and 1% (10mg) Aloe Polysaccharides. Other ingredients: Purified Water, Carbomer 980, Magnesium Aluminum Silicate, PEG-20 Methyl Glucose Ether, Aminomethyl Propanol 95, Biopeptide, Propylene Glycol, Glycerine, SDA Alcohol 40 B, Benzyl Alcohol, Trolamine, FD&C Blue #1 and D&C Yellow #10.
Iodoquinol
Iodoquinol is an antifungal and antibacterial agent. Chemically, Iodoquinol is [5,7-diiodo-8-quinolinol] with the molecular formula (C9H5I2NO) and is represented by the following structural formula:
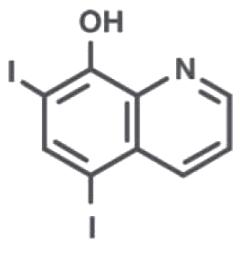
Aloe Polysaccharide
The Aloe Polysaccharide in ALOQUIN is a patented mixture of acetylated mannan aloe polysaccharide. Each purified acetylated mannan polysaccharide of specific molecular weight range and average is composed of the same repeating subunits shown below (where m is mannose, n is galactose and p is glucose monomers):
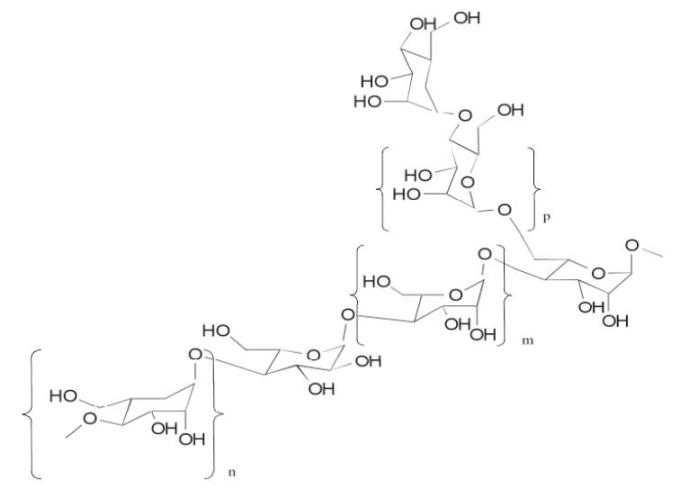
-
INDICATIONS AND USAGE
Based on a review of a related drug by the National Research Council and subsequent FDA classification for that drug, the indications are as follows: "Possibly" Effective: Contact or atopic dermatitis; impetiginized eczema; nummular eczema; endogenous chronic infectious dermatitis; stasis dermatitis; pyoderma; nuchal eczema and chronic eczematoid otitis externa; acne urticata; localized or disseminated neurodermatitis; lichen simplex chronicus; anogenital pruritus (vulvae, scroti, ani); folliculitis; bacterial dermatoses; mycotic dermatoses such as tinea (capitis, cruris, corporis, pedis); monliasis; intertrigo. Final classification of the less-than-effective indications requires further investigation.
- CONTRAINDICATIONS
-
WARNINGS AND PRECAUTIONS
For external use only. Keep away from eyes. If irritation develops, the use of ALOQUIN should be discontinued and appropriate therapy instituted. Some discoloration of the skin, hair and fabrics may occur, but can be removed with normal cleansing and laundry. Not intended for use on infants or under diapers or occlusive dressings.
Iodoquinol may be absorbed through the skin and interfere with thyroid function tests. If such tests are contemplated, wait at least one month after discontinuance of therapy to perform these tests. The ferric chloride test for phenylketonuria (PKU) can yield a false positive result if Iodoquinol is present in the diaper or urine. Prolonged use may result in overgrowth of non-susceptible organisms requiring appropriate therapy. Keep out of reach of children.
Carcinogenesis, Mutagenisis and Impairment of Fertility
Long term animal studies have not been performed to evaluate the carcinogenic potential of the effect on fertility of Iodoquinol. Mutagenicity studies have not been performed with Iodoquinol.
-
ADVERSE REACTIONS
Adverse reactions from topical use of ALOQUIN is expected to be low when used as directed, due to low concentration of Iodoquinol present in this topical gel.
To achieve the equivalent of a common daily oral dose of nearly 2,000 mg Iodoquinol, one will need to use more than 2 full tubes of 60 g ALOQUIN in a single application. Adverse reactions from oral form of Iodoquinol (nearly 2,000 mg daily) have been reported: various forms of skin eruptions, hives, itching, nausea, vomiting, abdominal cramps, diarrhea, anusitis, fever, chills, headache, vertigo and enlargement of thyroid.
- DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
-
SPL UNCLASSIFIED SECTION
Rx ONLY
www.aloquin.comManufactured for:
Primus Pharmaceuticals, Inc.
Scottsdale, AZ 85251
www.primusrx.comManufactured by:
Sonar Products, Inc.
Carlstadt, NJ 07072U.S. Patents #6,436,679; #6,271,214; #6,133,440; #5,925,357; #5,902,796; #5,708,038; #5,703,060; #5,468,737; other patents pending.
©2009 Primus Pharmaceuticals, Inc. All rights reserved.ISS.0309 #15905
- PRINCIPAL DISPLAY PANEL - 60g Carton
-
INGREDIENTS AND APPEARANCE
ALOQUIN
aloe vera leaf and iodoquinol gelProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:68040-706 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Aloe Vera Leaf (UNII: ZY81Z83H0X) (Aloe Vera Leaf - UNII:ZY81Z83H0X) Aloe Vera Leaf 10 mg in 1 g Iodoquinol (UNII: 63W7IE88K8) (Iodoquinol - UNII:63W7IE88K8) Iodoquinol 12.5 mg in 1 g Product Characteristics Color GREEN Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68040-706-16 1 in 1 BOX 1 60 g in 1 TUBE 2 NDC:68040-706-01 1 g in 1 PACKET 3 NDC:68040-706-08 10 in 1 BOX 3 1 g in 1 PACKET Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved other 07/06/2009 Labeler - Primus Pharmaceuticals (130834745) Establishment Name Address ID/FEI Business Operations Sonar Products, Inc 104283945 MANUFACTURE


