Label: NORITATE- metronidazole cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 54868-4449-1 - Packager: Physicians Total Care, Inc.
- This is a repackaged label.
- Source NDC Code(s): 0066-9850
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated May 6, 2011
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
NORITATE (metronidazole cream) Cream, 1%, contains metronidazole, USP. Chemically, metronidazole is 2-methyl-5-nitro-1H-imidazole-1-ethanol. The molecular formula for metronidazole is C6H9N3O3. It has the following structural formula:
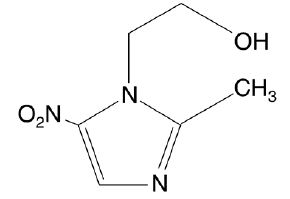
Metronidazole has a molecular weight of 171.16. It is a white to pale yellow crystalline powder. It is slightly soluble in alcohol and has a solubility in water of 10 mg/mL at 20°C. Metronidazole is a member of the imidazole class of antibacterial agents and is classified as an antiprotozoal and anti-bacterial agent.
NORITATE is an emollient cream; each gram contains 10 mg micronized metronidazole USP, in a base of purified water USP, stearic acid NF, glyceryl monostearate NF, glycerin USP, methylparaben NF, trolamine NF and propylparaben NF.
-
CLINICAL PHARMACOLOGY
Pharmacokinetics:
When one gram dose of NORITATE cream, 1%, was applied in a single application to the face of 16 healthy volunteers, low concentrations of metronidazole were detected in the plasma of 7 of the volunteers. The mean ± SD Cmax of metronidazole was 27.6 ± 7.3 ng/mL, which is about 1% of the value reported for a single 250 mg oral dose of metronidazole. The time to maximum plasma concentration (Tmax) in the volunteers with detectable metronidazole was 8-12 hoursafter topical application.
Pharmacodynamics:
The mechanisms by which metronidazole acts in reducing inflammatory lesions of rosacea are unknown.
Clinical Studies:
Safety and efficacy of NORITATE were evaluated in two randomized vehicle-controlled clinical studies for the treatment of rosacea, which excluded patients who had nodules, moderate or severe rhinophyma, dense telangiectases, plaque-like facial edema or ocular involvement and those who had a history of not responding to metronidazole therapy for rosacea. Of the patients
included in the efficacy database (n=416), there were 142 men and 274 women. Endpoint efficacy data comparisons for patients treated with daily NORITATE or vehicle applications are listed below.
* Statistically significant differences between NORITATE and vehicle groups with p<0.05. Erythema scores: 0=none, 1=mild, 2=moderate and 3=severe.Inflammatory Lesion Counts and Erythema Severity Scores in Two Clinical Trials for Rosacea
Nori tate
Vehi cle
Study 1 Study 2 Study 1 Study 2
N
Result
N
Result
N
Result
N
Result
Papules + Pustules Count
Baseline
89
15
92
19
50
18
49
17
Week-10
80
7*
82
8
45
15
41
12
Reduction
49%*
58%*
17%
30%
Papules Count
Baseline
89
13
92
17
50
15
49
15
Week-10
80
7*
82
7
45
12
41
11
Reduction
41%*
55%*
14%
28%
Erythema Score
Baseline
89
2.2
92
2.3
50
2.2
49
2.2
Week-10
80
1.3*
82
1.4*
45
1.7
40
1.8
Reduction
42%*
40%*
25%
19%
Safety Studies:
Studies of contact sensitization (n=258), phototoxicity (n=21), and photocontact sensitization (n=29) of NORITATE were conducted. No evidence of sensitization or phototoxicity was seen in these studies. - INDICATIONS AND USAGE
- CONTRAINDICATIONS
-
PRECAUTIONS
General:
If a reaction suggesting local skin irritation occurs, patients should be directed to discontinue use of the medication. Conjunctivitis associated with topical use of metronidazole on the face has been reported. Contact with the eyes should be avoided. Metronidazole is a nitroimidazole and should be used with care in patients with evidence of, or history of, blood dyscrasia.
Information for Patients:
Patients using NORITATE should receive the following information and instructions:
1. This medication is to be used as directed.
2. It is for external use only.
3. Avoid contact with the eyes.
4. Cleanse affected area(s) before applying NORITATE.
5. This medication should not be used for any disorder other than that for which it is prescribed.
6. Patients should report any adverse reaction to their physician.
Drug Interactions:
Oral metronidazole has been reported to potentiate the anticoagulant effect of coumarin and warfarin resulting in a prolongation of prothrombin time. Drug interactions should be kept in mind when NORITATE is prescribed for patients who are receiving anticoagulant treatment, although they are less likely to occur with topical metronidazole administration because of low absorption. (See CLINICAL PHARMACOLOGY, Pharmacokinetics section)
Carcinogenesis, Mutagenesis and Impairment of Fertility:
Metronidazole has shown evidence of carcinogenic activity in a number of studies involving chronic, oral administration in mice and rats but not in studies involving hamsters. In several long term studies in mice, oral doses of approximately 225 mg/m2/day or greater (approximately 37 times the human topical dose on a mg/m2 basis) were associated with an increase in pulmonary tumors and lymphomas. Several long term oral studies in the rat have shown statistically significant increases in mammary and hepatic tumors at doses >885 mg/m2/day (144 times the topical human dose).
Metronidazole has shown evidence of mutagenic activity in several in vitro bacterial assay systems. In addition, a dose-related increase in the frequency of micronuclei was observed in mice after intraperitoneal injections. An increase in chromosomal aberrations in peripheral blood lymphocytes was reported in patients with Crohn’s disease who were treated with 200 to 1200 mg/day of metronidazole for 1 to 24 months. However, in another study, no increase in chromosomal aberrations in circulating lymphocytes was observed in patients with Crohn’s disease treated with the drug for 8 months.
In one published study, using albino hairless mice, intraperitoneal administration of metronidazole at a dose of 45 mg/m2/day (approximately 7 times the human topical dose on a mg/m2 basis) was associated with an increase in ultraviolet radiationinduced skin carcinogenesis. Neither dermal carcinogenicity nor photocarcinogenicity studies have been performed with NORITATE or any marketed metronidazole
formulations.Pregnancy:
Teratogenic Effects: Pregnancy Category B. There are no adequate and well controlled studies with the use of NORITATE in pregnant women. Metronidazole crosses the placental barrier and enters the fetal circulation rapidly. No fetotoxicity was observed after oral administration of metronidazole to rats or mice at 200 and 20 times, respectively, the expected clinical dose. However, oral metronidazole has shown carcinogenic activity in rodents. Because animal reproduction studies are not always predictive of human response, NORITATE should be used during pregnancy only if clearly needed.
Nursing Mothers:
After oral administration, metronidazole is secreted in breast milk in concentrations similar to those found in the plasma. Even though blood levels taken after topical metronidazole application are significantly lower than those achieved after oral metronidazole, a decision should be made whether to discontinue the mother and the risk to the infant.
Pediatric Use:
Safety and effectiveness in pediatric patients have not been established.
-
ADVERSE REACTIONS
Safety data from 302 patients who used NORITATE (n=200) or vehicle control (n=102) once daily in clinical trials and experienced an adverse event considered to be treatment-related include: application site reaction (NORITATE 1, vehicle 1), condition aggravated (NORITATE 1, vehicle 0), paresthesia (NORITATE 0, vehicle 1), acne (NORITATE 1, vehicle 0), dry skin (NORITATE 0, vehicle 2). The majority of adverse reactions were mild to moderate in severity.
Two patients treated with NORITATE once daily discontinued treatment because of adverse events: one for a severe flare of comedonal acne and one for rosacea aggravated.
Additional clinical adverse effects reported spontaneously since the drug was marketed are uncommon and include tingling or numbness of extremities, allergic reactions, skin and eye irritation, rash, headache, nausea and dry mouth.
- DOSAGE AND ADMINISTRATION
-
HOW SUPPLIED
Cream - 60 gram aluminum tube NDC 54868-4449-1.
Keep out of the reach of children.
Storage Conditions: Store at controlled room temperature: 20 to 25°C (68 to 77°F).
Rx Only
Dermik Laboratories
a business of sanofi-aventis U.S. LLC
Bridgewater, NJ 08807
Revised December 2006
© 2006 sanofi-aventis U.S. LLC
Relabeling of "Additional" label by:
Physicians Total Care, Inc.
Tulsa, OK 74146
- PACKAGE LABEL PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
NORITATE
metronidazole creamProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:54868-4449(NDC:0066-9850) Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength METRONIDAZOLE (UNII: 140QMO216E) (METRONIDAZOLE - UNII:140QMO216E) METRONIDAZOLE 10 mg in 1 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) STEARIC ACID (UNII: 4ELV7Z65AP) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) GLYCERIN (UNII: PDC6A3C0OX) METHYLPARABEN (UNII: A2I8C7HI9T) TROLAMINE (UNII: 9O3K93S3TK) PROPYLPARABEN (UNII: Z8IX2SC1OH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54868-4449-1 1 in 1 CARTON 1 60 g in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA020743 09/29/2005 Labeler - Physicians Total Care, Inc. (194123980) Establishment Name Address ID/FEI Business Operations Physicians Total Care, Inc. 194123980 relabel


