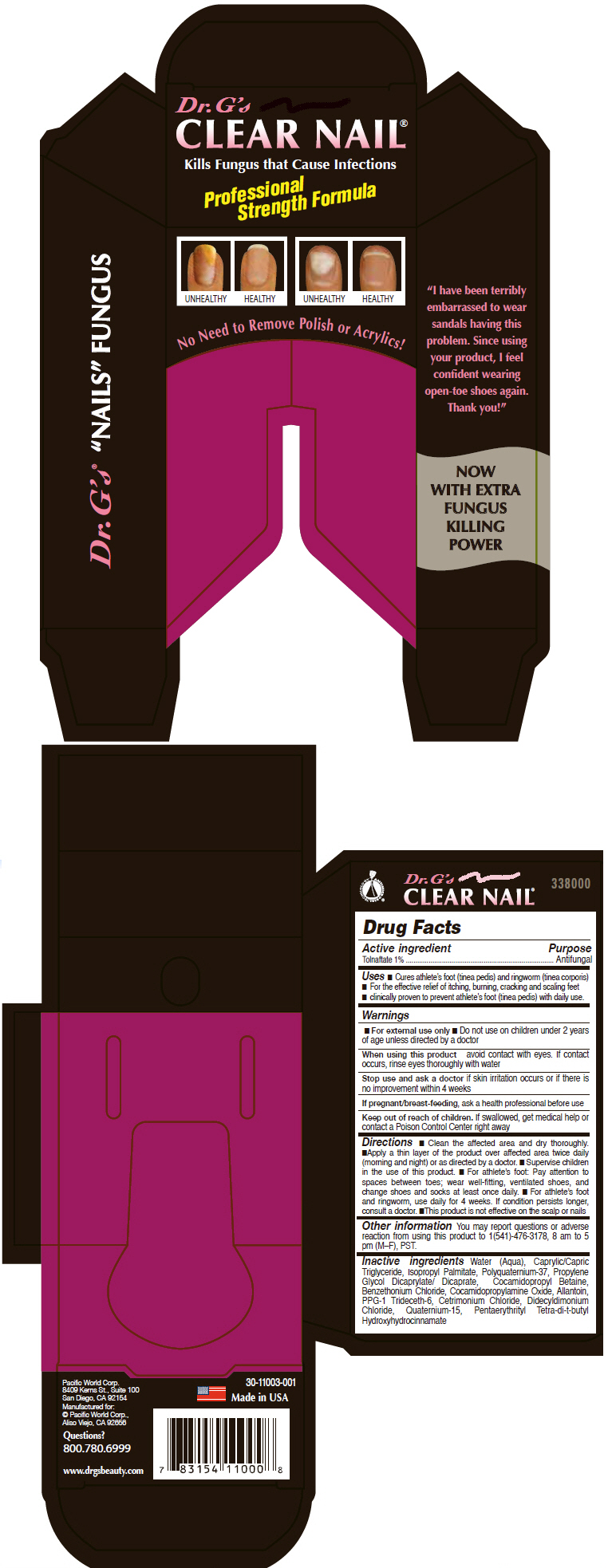
Label: DR. GS CLEAR NAIL- tolnaftate liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 60193-101-02 - Packager: Pacific World Corporation
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated October 17, 2014
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Uses
-
Warnings
For external use only.
Do not use
n near the mouth or eyes n with known sensitivities to any listed ingredients n on children under 2 years of age unless directed by a doctor
When using this product avoid eye contact. If accidental eye contact occurs, rinse thoroughly with water for 10-15 minutes.
-
Directions
n Clean affected area with soap and warm water and dry thoroughly n Apply a thin layer two times a day (mornings and evenings) to affected area especially the space between and around toes n For athlete's foot, use daily for four weeks or as directed by a physician n Allow solution to soak into skin or rub in to dry more quickly before putting on socks n This product is not effective on scalp or nails. n Following a proper foot hygiene regimen along with wearing well fitting, ventilated shoes and clean socks that are changed at least daily is helpful in preventing future infections
- Other information
- Inactive ingredients
- Questions?
- PRINCIPAL DISPLAY PANEL - 18 mL Tube Carton
-
INGREDIENTS AND APPEARANCE
DR. GS CLEAR NAIL
tolnaftate liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:60193-101 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TOLNAFTATE (UNII: 06KB629TKV) (TOLNAFTATE - UNII:06KB629TKV) TOLNAFTATE 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) ISOPROPYL PALMITATE (UNII: 8CRQ2TH63M) POLYQUATERNIUM-37 (3000 MPA.S) (UNII: HU373G0YSU) PROPYLENE GLYCOL DICAPRYLATE/DICAPRATE (UNII: O4446S9CRA) COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) BENZETHONIUM CHLORIDE (UNII: PH41D05744) COCAMIDOPROPYLAMINE OXIDE (UNII: M4SL82J7HK) ALLANTOIN (UNII: 344S277G0Z) PPG-1 TRIDECETH-6 (UNII: 1K7417JX6Q) CETRIMONIUM CHLORIDE (UNII: UC9PE95IBP) DIDECYLDIMONIUM CHLORIDE (UNII: JXN40O9Y9B) QUATERNIUM-15 (UNII: E40U03LEM0) PENTAERYTHRITOL TETRAKIS(3-(3,5-DI-TERT-BUTYL-4-HYDROXYPHENYL)PROPIONATE) (UNII: 255PIF62MS) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:60193-101-02 1 in 1 CARTON 1 18 mL in 1 TUBE, WITH APPLICATOR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part333C 04/24/2013 Labeler - Pacific World Corporation (089693097)