SAPHRIS- asenapine maleate tablet
Merck Sharp & Dohme Corp
----------
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use SAPHRIS (asenapine) safely and effectively. See full prescribing information for SAPHRIS.
SAPHRIS® (asenapine) sublingual tablets Initial U.S. Approval: 2009 WARNING: INCREASED MORTALITY IN ELDERLY PATIENTS WITH DEMENTIA-RELATED PSYCHOSISSee full prescribing information for complete boxed warning.Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. SAPHRIS is not approved for the treatment of patients with dementia-related psychosis. (5.1) INDICATIONS AND USAGESAPHRIS is an atypical antipsychotic indicated for:
DOSAGE AND ADMINISTRATION
Administration: Do not swallow tablet. SAPHRIS sublingual tablets should be placed under the tongue and left to dissolve completely. The tablet will dissolve in saliva within seconds. Eating and drinking should be avoided for 10 minutes after administration. (2.1, 17.1) DOSAGE FORMS AND STRENGTHSCONTRAINDICATIONSWARNINGS AND PRECAUTIONS
ADVERSE REACTIONSCommonly observed adverse reactions (incidence ≥5% and at least twice that for placebo) were (6.1):
To report SUSPECTED ADVERSE REACTIONS, contact Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., at 1-877-888-4231 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. DRUG INTERACTIONSUSE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION. Revised: 3/2013 |
FULL PRESCRIBING INFORMATION
WARNING: INCREASED MORTALITY IN ELDERLY PATIENTS WITH DEMENTIA-RELATED PSYCHOSIS
Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. Analyses of 17 placebo-controlled trials (modal duration of 10 weeks), largely in patients taking atypical antipsychotic drugs, revealed a risk of death in the drug-treated patients of between 1.6 to 1.7 times that seen in placebo-treated patients. Over the course of a typical 10-week controlled trial, the rate of death in drug-treated patients was about 4.5%, compared to a rate of about 2.6% in the placebo group. Although the causes of death were varied, most of the deaths appeared to be either cardiovascular (e.g., heart failure, sudden death) or infectious (e.g., pneumonia) in nature. Observational studies suggest that, similar to atypical antipsychotic drugs, treatment with conventional antipsychotic drugs may increase mortality. The extent to which the findings of increased mortality in observational studies may be attributed to the antipsychotic drug as opposed to some characteristic(s) of the patients is not clear. SAPHRIS® (asenapine) is not approved for the treatment of patients with dementia-related psychosis [see Warnings and Precautions (5.1)].
1 INDICATIONS AND USAGE
1.1 Schizophrenia
SAPHRIS is indicated for the treatment of schizophrenia. The efficacy of SAPHRIS was established in two 6-week trials and one maintenance trial in adults [see Clinical Studies (14.1)].
1.2 Bipolar Disorder
Monotherapy: SAPHRIS is indicated for the acute treatment of manic or mixed episodes associated with bipolar I disorder. Efficacy was established in two 3-week monotherapy trials in adults [see Clinical Studies (14.2)].
Adjunctive Therapy: SAPHRIS is indicated as adjunctive therapy with either lithium or valproate for the acute treatment of manic or mixed episodes associated with bipolar I disorder. Efficacy was established in one 3-week adjunctive trial in adults [see Clinical Studies (14.2)].
2 DOSAGE AND ADMINISTRATION
2.1 Administration Instructions
SAPHRIS is a sublingual tablet. To ensure optimal absorption, patients should be instructed to place the tablet under the tongue and allow it to dissolve completely. The tablet will dissolve in saliva within seconds. SAPHRIS sublingual tablets should not be crushed, chewed, or swallowed [see Clinical Pharmacology (12.3)]. Patients should be instructed to not eat or drink for 10 minutes after administration [see Clinical Pharmacology (12.3) and Patient Counseling Information (17.1)].
2.2 Schizophrenia
Usual Dose for Acute Treatment in Adults: The recommended starting and target dose of SAPHRIS is 5 mg given twice daily. In short term controlled trials, there was no suggestion of added benefit with a 10 mg twice daily dose, but there was a clear increase in certain adverse reactions. The safety of doses above 10 mg twice daily has not been evaluated in clinical studies.
Maintenance Treatment: Efficacy was demonstrated with SAPHRIS in a maintenance trial in patients with schizophrenia. The starting dose in this study was 5 mg twice daily with an increase up to 10 mg twice daily after 1 week based on tolerability [see Clinical Studies (14.1)]. While there is no body of evidence available to answer the question of how long the schizophrenic patient should remain on SAPHRIS, patients should be periodically reassessed to determine the need for maintenance treatment.
2.3 Bipolar Disorder
Usual Dose for Acute Treatment of Manic or Mixed Episodes Associated with Bipolar I Disorder in Adults:
Monotherapy: The recommended starting dose of SAPHRIS, and the dose maintained by 90% of the patients studied, is 10 mg twice daily. The dose can be decreased to 5 mg twice daily if warranted by adverse effects or based on individual tolerability.
In controlled monotherapy trials, the starting dose for SAPHRIS was 10 mg twice daily. On the second and subsequent days of the trials, the dose could be lowered to 5 mg twice daily, based on tolerability, but less than 10% of patients had their dose reduced. The safety of doses above 10 mg twice daily has not been evaluated in clinical trials.
Adjunctive Therapy: The recommended starting dose of SAPHRIS is 5 mg twice daily when administered as adjunctive therapy with either lithium or valproate. Depending on the clinical response and tolerability in the individual patient, the dose can be increased to 10 mg twice daily. The safety of doses above 10 mg twice daily as adjunctive therapy with lithium or valproate has not been evaluated in clinical trials.
Maintenance Treatment: While there is no body of evidence available to answer the question of how long the bipolar patient should remain on SAPHRIS, whether used as monotherapy or as adjunctive therapy with lithium or valproate, it is generally recommended that responding patients be continued beyond the acute response. If SAPHRIS is used for extended periods in bipolar disorder, the physician should periodically re-evaluate the long-term risks and benefits of the drug for the individual patient.
2.4 Dosage in Special Populations
In a study of subjects with hepatic impairment who were treated with a single dose of SAPHRIS 5 mg, there were increases in asenapine exposures (compared to subjects with normal hepatic function), that correlated with the degree of hepatic impairment. While the results indicated that no dosage adjustments are required in patients with mild (Child-Pugh A) or moderate (Child-Pugh B) hepatic impairment, there was a 7-fold increase (on average) in asenapine concentrations in subjects with severe hepatic impairment (Child-Pugh C) compared to the concentrations of those in subjects with normal hepatic function. Therefore, SAPHRIS is not recommended in patients with severe hepatic impairment [see Use in Special Populations (8.7)]. Dosage adjustments are not routinely required on the basis of age, gender, race, or renal impairment status [see Use in Specific Populations (8.4, 8.5, 8.6) and Clinical Pharmacology (12.3)].
2.5 Switching from Other Antipsychotics
There are no systematically collected data to specifically address switching patients with schizophrenia or bipolar mania from other antipsychotics to SAPHRIS or concerning concomitant administration with other antipsychotics. While immediate discontinuation of the previous antipsychotic treatment may be acceptable for some patients with schizophrenia, more gradual discontinuation may be most appropriate for others. In all cases, the period of overlapping antipsychotic administration should be minimized.
3 DOSAGE FORMS AND STRENGTHS
- SAPHRIS 5-mg tablets are round, white to off-white sublingual tablets, with "5" on one side.
- SAPHRIS 10-mg tablets are round, white to off-white sublingual tablets, with "10" on one side.
- SAPHRIS 5-mg tablets, black cherry flavor, are round, white to off-white sublingual tablets, with "5" on one side within a circle.
- SAPHRIS 10-mg tablets, black cherry flavor, are round, white to off-white sublingual tablets, with "10" on one side within a circle.
4 CONTRAINDICATIONS
Hypersensitivity reactions, including anaphylaxis and angioedema, have been observed in patients treated with asenapine. Therefore, SAPHRIS is contraindicated in patients with a known hypersensitivity to the product [see Warnings and Precautions (5.7), Adverse Reactions (6) and Patient Counseling Information (17.3)].
5 WARNINGS AND PRECAUTIONS
5.1 Increased Mortality in Elderly Patients with Dementia-Related Psychosis
Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. SAPHRIS is not approved for the treatment of patients with dementia-related psychosis [see Boxed Warning].
5.2 Cerebrovascular Adverse Events, Including Stroke, In Elderly Patients with Dementia-Related Psychosis
In placebo-controlled trials with risperidone, aripiprazole, and olanzapine in elderly subjects with dementia, there was a higher incidence of cerebrovascular adverse reactions (cerebrovascular accidents and transient ischemic attacks) including fatalities compared to placebo-treated subjects. SAPHRIS is not approved for the treatment of patients with dementia-related psychosis [see also Boxed Warning and Warnings and Precautions (5.1)].
5.3 Neuroleptic Malignant Syndrome
A potentially fatal symptom complex sometimes referred to as Neuroleptic Malignant Syndrome (NMS) has been reported in association with administration of antipsychotic drugs, including SAPHRIS. Clinical manifestations of NMS are hyperpyrexia, muscle rigidity, altered mental status, and evidence of autonomic instability (irregular pulse or blood pressure, tachycardia, diaphoresis, and cardiac dysrhythmia). Additional signs may include elevated creatine phosphokinase, myoglobinuria (rhabdomyolysis), and acute renal failure.
The diagnostic evaluation of patients with this syndrome is complicated. It is important to exclude cases where the clinical presentation includes both serious medical illness (e.g. pneumonia, systemic infection) and untreated or inadequately treated extrapyramidal signs and symptoms (EPS). Other important considerations in the differential diagnosis include central anticholinergic toxicity, heat stroke, drug fever, and primary central nervous system pathology.
The management of NMS should include: 1) immediate discontinuation of antipsychotic drugs and other drugs not essential to concurrent therapy; 2) intensive symptomatic treatment and medical monitoring; and 3) treatment of any concomitant serious medical problems for which specific treatments are available. There is no general agreement about specific pharmacological treatment regimens for NMS.
If a patient requires antipsychotic drug treatment after recovery from NMS, the potential reintroduction of drug therapy should be carefully considered. The patient should be carefully monitored, since recurrences of NMS have been reported.
5.4 Tardive Dyskinesia
A syndrome of potentially irreversible, involuntary, dyskinetic movements can develop in patients treated with antipsychotic drugs. Although the prevalence of the syndrome appears to be highest among the elderly, especially elderly women, it is impossible to rely upon prevalence estimates to predict, at the inception of antipsychotic treatment, which patients are likely to develop the syndrome. Whether antipsychotic drug products differ in their potential to cause Tardive Dyskinesia (TD) is unknown.
The risk of developing TD and the likelihood that it will become irreversible are believed to increase as the duration of treatment and the total cumulative dose of antipsychotic drugs administered to the patient increase. However, the syndrome can develop, although much less commonly, after relatively brief treatment periods at low doses.
There is no known treatment for established cases of TD, although the syndrome may remit, partially or completely, if antipsychotic treatment is withdrawn. Antipsychotic treatment, itself, however, may suppress (or partially suppress) the signs and symptoms of the syndrome and thereby may possibly mask the underlying process. The effect that symptomatic suppression has upon the long-term course of the syndrome is unknown.
Given these considerations, SAPHRIS should be prescribed in a manner that is most likely to minimize the occurrence of TD. Chronic antipsychotic treatment should generally be reserved for patients who suffer from a chronic illness that (1) is known to respond to antipsychotic drugs, and (2) for whom alternative, equally effective, but potentially less harmful treatments are not available or appropriate. In patients who do require chronic treatment, the smallest dose and the shortest duration of treatment producing a satisfactory clinical response should be sought. The need for continued treatment should be reassessed periodically.
If signs and symptoms of TD appear in a patient on SAPHRIS, drug discontinuation should be considered. However, some patients may require treatment with SAPHRIS despite the presence of the syndrome.
5.5 Hyperglycemia and Diabetes Mellitus
Hyperglycemia, in some cases extreme and associated with ketoacidosis or hyperosmolar coma or death, has been reported in patients treated with atypical antipsychotics. Assessment of the relationship between atypical antipsychotic use and glucose abnormalities is complicated by the possibility of an increased background risk of diabetes mellitus in patients with schizophrenia and the increasing incidence of diabetes mellitus in the general population. Given these confounders, the relationship between atypical antipsychotic use and hyperglycemia-related adverse reactions is not completely understood. However, epidemiological studies suggest an increased risk of treatment-emergent hyperglycemia-related adverse events in patients treated with the atypical antipsychotics included in these studies. Because SAPHRIS was not marketed at the time these studies were performed, it is not known if SAPHRIS is associated with this increased risk. Precise risk estimates for hyperglycemia-related adverse events in patients treated with atypical antipsychotics are not available.
Patients with an established diagnosis of diabetes mellitus who are started on atypical antipsychotics should be monitored regularly for worsening of glucose control. Patients with risk factors for diabetes mellitus (e.g., obesity, family history of diabetes) who are starting treatment with atypical antipsychotics should undergo fasting blood glucose testing at the beginning of treatment and periodically during treatment. Any patient treated with atypical antipsychotics should be monitored for symptoms of hyperglycemia including polydipsia, polyuria, polyphagia, and weakness. Patients who develop symptoms of hyperglycemia during treatment with atypical antipsychotics should undergo fasting blood glucose testing. In some cases, hyperglycemia has resolved when the atypical antipsychotic was discontinued; however, some patients required continuation of anti-diabetic treatment despite discontinuation of the antipsychotic drug.
5.6 Weight Gain
Increases in weight have been observed in pre-marketing clinical trials with SAPHRIS. Patients receiving SAPHRIS should receive regular monitoring of weight [see Patient Counseling Information (17.7)].
In short-term schizophrenia and bipolar mania trials, there were differences in mean weight gain between SAPHRIS-treated and placebo-treated patients. In short-term, placebo-controlled schizophrenia trials, the mean weight gain was 1.1 kg for SAPHRIS-treated patients compared to 0.1 kg for placebo-treated patients. The proportion of patients with a ≥7% increase in body weight (at Endpoint) was 4.9% for SAPHRIS-treated patients versus 2% for placebo-treated patients. In short-term, placebo-controlled bipolar mania trials, the mean weight gain for SAPHRIS-treated patients was 1.3 kg compared to 0.2 kg for placebo-treated patients. The proportion of patients with a ≥7% increase in body weight (at Endpoint) was 5.8% for SAPHRIS-treated patients versus 0.5% for placebo-treated patients.
In a 52-week, double-blind, comparator-controlled trial of patients with schizophrenia or schizoaffective disorder, the mean weight gain from baseline was 0.9 kg. The proportion of patients with a ≥7% increase in body weight (at Endpoint) was 14.7%. Table 1 provides the mean weight change from baseline and the proportion of patients with a weight gain of ≥7% categorized by Body Mass Index (BMI) at baseline:
| BMI <23 SAPHRIS N=295 | BMI 23 - ≤27 SAPHRIS N=290 | BMI >27 SAPHRIS N=302 |
|
|---|---|---|---|
| Mean change from Baseline (kg) | 1.7 | 1 | 0 |
| % with ≥7% increase in body weight | 22% | 13% | 9% |
5.7 Hypersensitivity Reactions
Hypersensitivity reactions, including anaphylaxis and angioedema, have been observed in patients treated with asenapine. In several cases, these reactions occurred after the first dose. These hypersensitivity reactions included: anaphylaxis, angioedema, hypotension, tachycardia, swollen tongue, dyspnea, wheezing and rash.
5.8 Orthostatic Hypotension, Syncope, and Other Hemodynamic Effects
SAPHRIS may induce orthostatic hypotension and syncope in some patients, especially early in treatment, because of its α1-adrenergic antagonist activity. In short-term schizophrenia trials, syncope was reported in 0.2% (1/572) of patients treated with therapeutic doses (5 mg or 10 mg twice daily) of SAPHRIS, compared to 0.3% (1/378) of patients treated with placebo. In short-term bipolar mania trials, syncope was reported in 0.3% (1/379) of patients treated with therapeutic doses (5 mg or 10 mg twice daily) of SAPHRIS, compared to 0% (0/203) of patients treated with placebo. During pre-marketing clinical trials with SAPHRIS, including long-term trials without comparison to placebo, syncope was reported in 0.6% (11/1953) of patients treated with SAPHRIS.
Four normal volunteers in clinical pharmacology studies treated with either intravenous, oral, or sublingual SAPHRIS experienced hypotension, bradycardia, and sinus pauses. These spontaneously resolved in 3 cases, but the fourth subject received external cardiac massage. The risk of this sequence of hypotension, bradycardia, and sinus pause might be greater in nonpsychiatric patients compared to psychiatric patients who are possibly more adapted to certain effects of psychotropic drugs.
Patients should be instructed about nonpharmacologic interventions that help to reduce the occurrence of orthostatic hypotension (e.g., sitting on the edge of the bed for several minutes before attempting to stand in the morning and slowly rising from a seated position). SAPHRIS should be used with caution in (1) patients with known cardiovascular disease (history of myocardial infarction or ischemic heart disease, heart failure or conduction abnormalities), cerebrovascular disease, or conditions which would predispose patients to hypotension (dehydration, hypovolemia, and treatment with antihypertensive medications); and (2) in the elderly. SAPHRIS should be used cautiously when treating patients who receive treatment with other drugs that can induce hypotension, bradycardia, respiratory or central nervous system depression [see Drug Interactions (7)]. Monitoring of orthostatic vital signs should be considered in all such patients, and a dose reduction should be considered if hypotension occurs.
5.9 Leukopenia, Neutropenia, and Agranulocytosis
In clinical trial and postmarketing experience, events of leukopenia/neutropenia have been reported temporally related to antipsychotic agents, including SAPHRIS. Agranulocytosis (including fatal cases) has been reported with other agents in the class.
Possible risk factors for leukopenia/neutropenia include pre-existing low white blood cell count (WBC) and history of drug induced leukopenia/neutropenia. Patients with a pre-existing low WBC or a history of drug induced leukopenia/neutropenia should have their complete blood count (CBC) monitored frequently during the first few months of therapy and SAPHRIS should be discontinued at the first sign of decline in WBC in the absence of other causative factors.
Patients with neutropenia should be carefully monitored for fever or other symptoms or signs of infection and treated promptly if such symptoms or signs occur. Patients with severe neutropenia (absolute neutrophil count <1000/mm3) should discontinue SAPHRIS and have their WBC followed until recovery.
5.10 QT Prolongation
The effects of SAPHRIS on the QT/QTc interval were evaluated in a dedicated QT study. This trial involved SAPHRIS doses of 5 mg, 10 mg, 15 mg, and 20 mg twice daily, and placebo, and was conducted in 151 clinically stable patients with schizophrenia, with electrocardiographic assessments throughout the dosing interval at baseline and steady state. At these doses, SAPHRIS was associated with increases in QTc interval ranging from 2 to 5 msec compared to placebo. No patients treated with SAPHRIS experienced QTc increases ≥60 msec from baseline measurements, nor did any patient experience a QTc of ≥500 msec.
Electrocardiogram (ECG) measurements were taken at various time points during the SAPHRIS clinical trial program (5-mg or 10-mg twice daily doses). Post-baseline QT prolongations exceeding 500 msec were reported at comparable rates for SAPHRIS and placebo in these short-term trials. There were no reports of Torsade de Pointes or any other adverse reactions associated with delayed ventricular repolarization.
The use of SAPHRIS should be avoided in combination with other drugs known to prolong QTc including Class 1A antiarrhythmics (e.g., quinidine, procainamide) or Class 3 antiarrhythmics (e.g., amiodarone, sotalol), antipsychotic medications (e.g., ziprasidone, chlorpromazine, thioridazine), and antibiotics (e.g., gatifloxacin, moxifloxacin). SAPHRIS should also be avoided in patients with a history of cardiac arrhythmias and in other circumstances that may increase the risk of the occurrence of torsade de pointes and/or sudden death in association with the use of drugs that prolong the QTc interval, including bradycardia; hypokalemia or hypomagnesemia; and presence of congenital prolongation of the QT interval.
5.11 Hyperprolactinemia
Like other drugs that antagonize dopamine D2 receptors, SAPHRIS can elevate prolactin levels, and the elevation can persist during chronic administration. Hyperprolactinemia may suppress hypothalamic GnRH, resulting in reduced pituitary gonadotropin secretion. This, in turn, may inhibit reproductive function by impairing gonadal steroidogenesis in both female and male patients. Galactorrhea, amenorrhea, gynecomastia, and impotence have been reported in patients receiving prolactin-elevating compounds. Long-standing hyperprolactinemia when associated with hypogonadism may lead to decreased bone density in both female and male subjects. In SAPHRIS clinical trials, the incidences of adverse events related to abnormal prolactin levels were 0.4% versus 0% for placebo [see Adverse Reactions (6.1)].
Tissue culture experiments indicate that approximately one-third of human breast cancers are prolactin-dependent in vitro, a factor of potential importance if the prescription of these drugs is considered in a patient with previously-detected breast cancer. Neither clinical studies nor epidemiologic studies conducted to date have shown an association between chronic administration of this class of drugs and tumorigenesis in humans, but the available evidence is too limited to be conclusive.
5.12 Seizures
Seizures were reported in 0% and 0.3% (0/572, 1/379) of patients treated with doses of 5 mg and 10 mg twice daily of SAPHRIS, respectively, compared to 0% (0/503, 0/203) of patients treated with placebo in short-term schizophrenia and bipolar mania trials, respectively. During pre-marketing clinical trials with SAPHRIS, including long-term trials without comparison to placebo, seizures were reported in 0.3% (5/1953) of patients treated with SAPHRIS. As with other antipsychotic drugs, SAPHRIS should be used with caution in patients with a history of seizures or with conditions that potentially lower the seizure threshold, e.g., Alzheimer's dementia. Conditions that lower the seizure threshold may be more prevalent in patients 65 years or older.
5.13 Potential for Cognitive and Motor Impairment
Somnolence was reported in patients treated with SAPHRIS. It was usually transient with the highest incidence reported during the first week of treatment. In short-term, fixed-dose, placebo-controlled schizophrenia trials, somnolence was reported in 15% (41/274) of patients on SAPHRIS 5 mg twice daily and in 13% (26/208) of patients on SAPHRIS 10 mg twice daily compared to 7% (26/378) of placebo patients. In short-term, placebo-controlled bipolar mania trials of therapeutic doses (5-10 mg twice daily), somnolence was reported in 24% (90/379) of patients on SAPHRIS compared to 6% (13/203) of placebo patients. During pre-marketing clinical trials with SAPHRIS, including long-term trials without comparison to placebo, somnolence was reported in 18% (358/1953) of patients treated with SAPHRIS. Somnolence (including sedation) led to discontinuation in 0.6% (12/1953) of patients in short-term, placebo-controlled trials.
Patients should be cautioned about performing activities requiring mental alertness, such as operating hazardous machinery or operating a motor vehicle, until they are reasonably certain that SAPHRIS therapy does not affect them adversely.
5.14 Body Temperature Regulation
Disruption of the body's ability to reduce core body temperature has been attributed to antipsychotic agents. In the short-term placebo-controlled trials for both schizophrenia and acute bipolar disorder, the incidence of adverse reactions suggestive of body temperature increases was low (≤1%) and comparable to placebo. During pre-marketing clinical trials with SAPHRIS, including long-term trials without comparison to placebo, the incidence of adverse reactions suggestive of body temperature increases (pyrexia and feeling hot) was ≤1%. Appropriate care is advised when prescribing SAPHRIS for patients who will be experiencing conditions that may contribute to an elevation in core body temperature, e.g., exercising strenuously, exposure to extreme heat, receiving concomitant medication with anticholinergic activity, or being subject to dehydration.
5.15 Suicide
The possibility of a suicide attempt is inherent in psychotic illnesses and bipolar disorder, and close supervision of high-risk patients should accompany drug therapy. Prescriptions for SAPHRIS should be written for the smallest quantity of tablets consistent with good patient management in order to reduce the risk of overdose.
5.16 Dysphagia
Esophageal dysmotility and aspiration have been associated with antipsychotic drug use. Dysphagia was reported in 0.2% and 0% (1/572, 0/379) of patients treated with therapeutic doses (5-10 mg twice daily) of SAPHRIS as compared to 0% (0/378, 0/203) of patients treated with placebo in short-term schizophrenia and bipolar mania trials, respectively. During pre-marketing clinical trials with SAPHRIS, including long-term trials without comparison to placebo, dysphagia was reported in 0.1% (2/1953) of patients treated with SAPHRIS.
Aspiration pneumonia is a common cause of morbidity and mortality in elderly patients, in particular those with advanced Alzheimer's dementia. SAPHRIS is not indicated for the treatment of dementia-related psychosis, and should not be used in patients at risk for aspiration pneumonia [see also Warnings and Precautions (5.1)].
5.17 Use in Patients with Concomitant Illness
Clinical experience with SAPHRIS in patients with certain concomitant systemic illnesses is limited [see Clinical Pharmacology (12.3)].
SAPHRIS has not been evaluated in patients with a recent history of myocardial infarction or unstable heart disease. Patients with these diagnoses were excluded from pre-marketing clinical trials. Because of the risk of orthostatic hypotension with SAPHRIS, caution should be observed in cardiac patients [see Warnings and Precautions (5.6)].
6 ADVERSE REACTIONS
The following adverse reactions are discussed in more detail in other sections of the labeling:
- Use in Elderly Patients with Dementia-Related Psychosis [see Boxed Warning and Warnings and Precautions (5.1 and 5.2)]
- Neuroleptic Malignant Syndrome [see Warnings and Precautions (5.3)]
- Tardive Dyskinesia [see Warnings and Precautions (5.4)]
- Hyperglycemia and Diabetes Mellitus [see Warnings and Precautions (5.5)]
- Weight Gain [see Warnings and Precautions (5.6)]
- Hypersensitivity Reactions [see Warnings and Precautions (5.7) and Patient Counseling Information (17.3)]
- Application site reactions including oral ulcers, blisters, peeling/sloughing and inflammation [see Adverse Reactions (6.2)]
- Orthostatic Hypotension, Syncope, and other Hemodynamic Effects [see Warnings and Precautions (5.8)]
- Leukopenia, Neutropenia, and Agranulocytosis [see Warnings and Precautions (5.9)]
- QT Interval Prolongation [see Warnings and Precautions (5.10)]
- Hyperprolactinemia [see Warnings and Precautions (5.11)]
- Seizures [see Warnings and Precautions (5.12)]
- Potential for Cognitive and Motor Impairment [see Warnings and Precautions (5.13)]
- Body Temperature Regulation [see Warnings and Precautions (5.14)]
- Suicide [see Warnings and Precautions (5.15)]
- Dysphagia [see Warnings and Precautions (5.16)]
- Use in Patients with Concomitant Illness [see Warnings and Precautions (5.17)]
The most common adverse reactions (≥5% and at least twice the rate of placebo) reported with acute treatment in schizophrenia were akathisia, oral hypoesthesia, and somnolence. The safety profile of SAPHRIS in the maintenance treatment of schizophrenia was similar to that seen with acute treatment.
The most common adverse reactions (≥5% and at least twice the rate of placebo) reported with acute monotherapy treatment of manic or mixed episodes associated with bipolar I disorder were somnolence, dizziness, extrapyramidal symptoms other than akathisia, and weight increased and during the adjunctive therapy trial in bipolar disorder were somnolence and oral hypoesthesia.
The information below is derived from a clinical trial database for SAPHRIS consisting of over 4565 patients and/or normal subjects exposed to one or more sublingual doses of SAPHRIS. A total of 1314 SAPHRIS-treated patients were treated for at least 24 weeks and 785 SAPHRIS-treated patients had at least 52 weeks of exposure at therapeutic doses.
The stated frequencies of adverse reactions represent the proportion of individuals who experienced a treatment-emergent adverse event of the type listed. A reaction was considered treatment emergent if it occurred for the first time or worsened while receiving therapy following baseline evaluation.
The figures in the tables and tabulations cannot be used to predict the incidence of side effects in the course of usual medical practice where patient characteristics and other factors differ from those that prevailed in the clinical trials. Similarly, the cited frequencies cannot be compared with figures obtained from other clinical investigations involving different treatment, uses, and investigators. The cited figures, however, do provide the prescriber with some basis for estimating the relative contribution of drug and nondrug factors to the adverse reaction incidence in the population studied.
6.1 Clinical Studies Experience
Adult Patients with Schizophrenia: The following findings are based on the short-term placebo-controlled pre-marketing trials for schizophrenia (a pool of three 6-week fixed-dose trials and one 6-week flexible-dose trial) in which sublingual SAPHRIS was administered in doses ranging from 5 to 10 mg twice daily.
Adverse Reactions Associated with Discontinuation of Treatment: A total of 9% of SAPHRIS-treated subjects and 10% of placebo subjects discontinued due to adverse reactions. There were no drug-related adverse reactions associated with discontinuation in subjects treated with SAPHRIS at the rate of at least 1% and at least twice the placebo rate.
Adverse Reactions Occurring at an Incidence of 2% or More in SAPHRIS-Treated Schizophrenic Patients: Adverse reactions associated with the use of SAPHRIS (incidence of 2% or greater, rounded to the nearest percent, and SAPHRIS incidence greater than placebo) that occurred during acute therapy (up to 6-weeks in patients with schizophrenia) are shown in Table 2.
| System Organ Class/ Preferred Term | Placebo N=378 | SAPHRIS 5 mg twice daily N=274 | SAPHRIS 10 mg twice daily N=208 | All SAPHRIS*
5 mg or 10 mg twice daily N=572 |
|---|---|---|---|---|
|
||||
| Gastrointestinal disorders | ||||
| Constipation | 6% | 7% | 4% | 5% |
| Dry mouth | 1% | 3% | 1% | 2% |
| Oral hypoesthesia | 1% | 6% | 7% | 5% |
| Salivary hypersecretion | 0% | <1% | 4% | 2% |
| Stomach discomfort | 1% | <1% | 3% | 2% |
| Vomiting | 5% | 4% | 7% | 5% |
| General disorders | ||||
| Fatigue | 3% | 4% | 3% | 3% |
| Irritability | <1% | 2% | 1% | 2% |
| Investigations | ||||
| Weight increased | <1% | 2% | 2% | 3% |
| Metabolism disorders | ||||
| Increased appetite | <1% | 3% | 0% | 2% |
| Nervous system disorders | ||||
| Akathisia† | 3% | 4% | 11% | 6% |
| Dizziness | 4% | 7% | 3% | 5% |
| Extrapyramidal symptoms (excluding akathisia)‡ | 7% | 9% | 12% | 10% |
| Somnolence§ | 7% | 15% | 13% | 13% |
| Psychiatric disorders | ||||
| Insomnia | 13% | 16% | 15% | 15% |
| Vascular disorders | ||||
| Hypertension | 2% | 2% | 3% | 2% |
Monotherapy in Adult Patients with Bipolar Mania: The following findings are based on the short-term placebo-controlled trials for bipolar mania (a pool of two 3-week flexible-dose trials) in which sublingual SAPHRIS was administered in doses of 5 mg or 10 mg twice daily.
Adverse Reactions Associated with Discontinuation of Treatment: Approximately 10% (38/379) of SAPHRIS-treated patients in short-term, placebo-controlled trials discontinued treatment due to an adverse reaction, compared with about 6% (12/203) on placebo. The most common adverse reactions associated with discontinuation in subjects treated with SAPHRIS (rates at least 1% and at least twice the placebo rate) were anxiety (1.1%) and oral hypoesthesia (1.1%) compared to placebo (0%).
Adverse Reactions Occurring at an Incidence of 2% or More Among SAPHRIS-Treated (Monotherapy) Bipolar Patients: Adverse reactions associated with the use of SAPHRIS (incidence of 2% or greater, rounded to the nearest percent, and SAPHRIS incidence greater than placebo) that occurred during acute monotherapy (up to 3-weeks in patients with bipolar mania) are shown in Table 3.
| System Organ Class/Preferred Term | Placebo N=203 | SAPHRIS 5 mg or 10 mg twice daily* N=379 |
|---|---|---|
|
||
| Gastrointestinal disorders | ||
| Dry mouth | 1% | 3% |
| Dyspepsia | 2% | 4% |
| Oral hypoesthesia | <1% | 4% |
| Toothache | 2% | 3% |
| General disorders | ||
| Fatigue | 2% | 4% |
| Investigations | ||
| Weight increased | <1% | 5% |
| Metabolism disorders | ||
| Increased appetite | 1% | 4% |
| Musculoskeletal and connective tissue disorders | ||
| Arthralgia | 1% | 3% |
| Pain in extremity | <1% | 2% |
| Nervous system disorders | ||
| Akathisia | 2% | 4% |
| Dizziness | 3% | 11% |
| Dysgeusia | <1% | 3% |
| Headache | 11% | 12% |
| Other extrapyramidal symptoms (excluding akathisia)† | 2% | 7% |
| Somnolence‡ | 6% | 24% |
| Psychiatric disorders | ||
| Anxiety | 2% | 4% |
| Depression | 1% | 2% |
| Insomnia | 5% | 6% |
Adjunctive Therapy in Adult Patients with Bipolar Mania: The following findings are based on a 12 week placebo-controlled trial (with a 3 week efficacy endpoint) in adult patients with bipolar mania in which sublingual SAPHRIS was administered in doses of 5 mg or 10 mg twice daily as adjunctive therapy with lithium or valproate.
Adverse Reactions Associated with Discontinuation of Treatment: Approximately 16% (25/158) of SAPHRIS-treated patients discontinued treatment due to an adverse reaction, compared with about 11% (18/166) on placebo. The most common adverse reactions associated with discontinuation in subjects treated with SAPHRIS (rates at least 1% and at least twice the placebo rate) were depression (2.5%), suicidal ideation (2.5%), bipolar 1 disorder (1.9%), insomnia (1.9%) and depressive symptoms (1.3%).
Adverse Reactions Occurring at an Incidence of 2% or More Among SAPHRIS-Treated (Adjunctive) Bipolar Patients: Adverse reactions associated with the use of SAPHRIS (incidence of 2% or greater, rounded to the nearest percent, and SAPHRIS incidence greater than placebo) that occurred during acute adjunctive therapy at 3 weeks, a time when most of the patients were still participating in the trial, are shown in Table 4.
| System Organ Class/Preferred Term | Placebo N=166 | SAPHRIS 5 mg or 10 mg twice daily* N=158 |
|---|---|---|
| Gastrointestinal disorders | ||
| Dyspepsia | 2% | 3% |
| Oral hypoesthesia | 0% | 5% |
| General disorders | ||
| Fatigue | 2% | 4% |
| Edema peripheral | <1% | 3% |
| Investigations | ||
| Weight increased | 0% | 3% |
| Nervous system disorders | ||
| Dizziness | 2% | 4% |
| Other extrapyramidal symptoms (excluding akathisia)† | 5% | 6% |
| Somnolence‡ | 10% | 22% |
| Psychiatric disorders | ||
| Insomnia | 8% | 10% |
| Vascular disorders | ||
| Hypertension | <1% | 3% |
Dystonia: Antipsychotic Class Effect: Symptoms of dystonia, prolonged abnormal contractions of muscle groups, may occur in susceptible individuals during the first few days of treatment. Dystonic symptoms include: spasm of the neck muscles, sometimes progressing to tightness of the throat, swallowing difficulty, difficulty breathing, and/or protrusion of the tongue. While these symptoms can occur at low doses, they occur more frequently and with greater severity with high potency and at higher doses of first generation antipsychotic drugs. An elevated risk of acute dystonia is observed in males and younger age groups.
Extrapyramidal Symptoms: In the short-term, placebo-controlled schizophrenia and bipolar mania trials, data was objectively collected on the Simpson Angus Rating Scale for extrapyramidal symptoms (EPS), the Barnes Akathisia Scale (for akathisia) and the Assessments of Involuntary Movement Scales (for dyskinesias). The mean change from baseline for the all-SAPHRIS 5 mg or 10 mg twice daily treated group was comparable to placebo in each of the rating scale scores.
In the short-term, placebo-controlled schizophrenia trials, the incidence of reported EPS-related events, excluding events related to akathisia, for SAPHRIS-treated patients was 10% versus 7% for placebo; and the incidence of akathisia-related events for SAPHRIS-treated patients was 6% versus 3% for placebo. In short-term placebo-controlled bipolar mania trials, the incidence of EPS-related events, excluding events related to akathisia, for SAPHRIS-treated patients was 7% versus 2% for placebo; and the incidence of akathisia-related events for SAPHRIS-treated patients was 4% versus 2% for placebo.
Other Findings: Oral hypoesthesia and/or oral paraesthesia may occur directly after administration of asenapine and usually resolves within 1 hour.
Laboratory Test Abnormalities:
Glucose: The effects on fasting serum glucose levels in the short-term schizophrenia and bipolar mania trials revealed no clinically relevant mean changes [see also Warnings and Precautions (5.5)]. In the short-term placebo-controlled schizophrenia trials, the mean increase in fasting glucose levels for SAPHRIS-treated patients was 3.2 mg/dL compared to a decrease of 1.6 mg/dL for placebo-treated patients. The proportion of patients with fasting glucose elevations ≥126 mg/dL (at Endpoint), was 7.4% for SAPHRIS-treated patients versus 6% for placebo-treated patients. In the short-term, placebo-controlled bipolar mania trials, the mean decreases in fasting glucose levels for both SAPHRIS-treated and placebo-treated patients were 0.6 mg/dL. The proportion of patients with fasting glucose elevations ≥126 mg/dL (at Endpoint), was 4.9% for SAPHRIS-treated patients versus 2.2% for placebo-treated patients.
In a 52-week, double-blind, comparator-controlled trial of patients with schizophrenia and schizoaffective disorder, the mean increase from baseline of fasting glucose was 2.4 mg/dL.
Lipids: The effects on total cholesterol and fasting triglycerides in the short-term schizophrenia and bipolar mania trials revealed no clinically relevant mean changes. In short-term, placebo-controlled schizophrenia trials, the mean increase in total cholesterol levels for SAPHRIS-treated patients was 0.4 mg/dL compared to a decrease of 3.6 mg/dL for placebo-treated patients. The proportion of patients with total cholesterol elevations ≥240 mg/dL (at Endpoint) was 8.3% for SAPHRIS-treated patients versus 7% for placebo-treated patients. In short-term, placebo-controlled bipolar mania trials, the mean increase in total cholesterol levels for SAPHRIS-treated patients was 1.1 mg/dL compared to a decrease of 1.5 mg/dL in placebo-treated patients. The proportion of patients with total cholesterol elevations ≥240 mg/dL (at Endpoint) was 8.7% for SAPHRIS-treated patients versus 8.6% for placebo-treated patients. In short-term, placebo-controlled schizophrenia trials, the mean increase in triglyceride levels for SAPHRIS-treated patients was 3.8 mg/dL compared to a decrease of 13.5 mg/dL for placebo-treated patients. The proportion of patients with elevations in triglycerides ≥200 mg/dL (at Endpoint) was 13.2% for SAPHRIS-treated patients versus 10.5% for placebo-treated patients. In short-term, placebo-controlled bipolar mania trials, the mean decrease in triglyceride levels for SAPHRIS-treated patients was 3.5 mg/dL versus 17.9 mg/dL for placebo-treated subjects. The proportion of patients with elevations in triglycerides ≥200 mg/dL (at Endpoint) was 15.2% for SAPHRIS-treated patients versus 11.4% for placebo-treated patients.
In a 52-week, double-blind, comparator-controlled trial of patients with schizophrenia and schizoaffective disorder, the mean decrease from baseline of total cholesterol was 6 mg/dL and the mean decrease from baseline of fasting triglycerides was 9.8 mg/dL.
Transaminases: Transient elevations in serum transaminases (primarily ALT) in the short-term schizophrenia and bipolar mania trials were more common in treated patients but mean changes were not clinically relevant. In short-term, placebo-controlled schizophrenia trials, the mean increase in transaminase levels for SAPHRIS-treated patients was 1.6 units/L compared to a decrease of 0.4 units/L for placebo-treated patients. The proportion of patients with transaminase elevations ≥3 times ULN (at Endpoint) was 0.9% for SAPHRIS-treated patients versus 1.3% for placebo-treated patients. In short-term, placebo-controlled bipolar mania trials, the mean increase in transaminase levels for SAPHRIS-treated patients was 8.9 units/L compared to a decrease of 4.9 units/L in placebo-treated patients. The proportion of patients with transaminase elevations ≥3 times upper limit of normal (ULN) (at Endpoint) was 2.5% for SAPHRIS-treated patients versus 0.6% for placebo-treated patients. No cases of more severe liver injury were seen.
In a 52-week, double-blind, comparator-controlled trial of patients with schizophrenia and schizoaffective disorder, the mean increase from baseline of ALT was 1.7 units/L.
Prolactin: The effects on prolactin levels in the short-term schizophrenia and bipolar mania trials revealed no clinically relevant mean changes in baseline. In short-term, placebo-controlled schizophrenia trials, the mean decreases in prolactin levels were 6.5 ng/mL for SAPHRIS-treated patients compared to 10.7 ng/mL for placebo-treated patients. The proportion of patients with prolactin elevations ≥4 times ULN (at Endpoint) were 2.6% for SAPHRIS-treated patients versus 0.6% for placebo-treated patients. In short-term, placebo-controlled bipolar mania trials, the mean increase in prolactin levels was 4.9 ng/mL for SAPHRIS-treated patients compared to a decrease of 0.2 ng/mL for placebo-treated patients. The proportion of patients with prolactin elevations ≥4 times ULN (at Endpoint) were 2.3% for SAPHRIS-treated patients versus 0.7% for placebo-treated patients.
In a long-term (52-week), double-blind, comparator-controlled trial of patients with schizophrenia and schizoaffective disorder, the mean decrease in prolactin from baseline for SAPHRIS-treated patients was 26.9 ng/mL.
Creatine Kinase (CK): The proportion of patients with CK elevations >3 times ULN at any time were 6.4% and 11.1% for patients treated with SAPHRIS 5 mg bid and 10 mg bid, respectively, as compared to 6.7% for placebo-treated patients in short-term, fixed-dose trials in schizophrenia and bipolar mania. The clinical relevance of this finding is unknown.
Other Adverse Reactions Observed During the Premarketing Evaluation of SAPHRIS: Following is a list of MedDRA terms that reflect adverse reactions reported by patients treated with sublingual SAPHRIS at multiple doses of ≥5 mg twice daily during any phase of a trial within the database of adult patients. The reactions listed are those that could be of clinical importance, as well as reactions that are plausibly drug-related on pharmacologic or other grounds. Reactions already listed in other parts of Adverse Reactions (6), or those considered in Warnings and Precautions (5) or Overdosage (10) are not included. Although the reactions reported occurred during treatment with SAPHRIS, they were not necessarily caused by it. Reactions are further categorized by MedDRA system organ class and listed in order of decreasing frequency according to the following definitions: those occurring in at least 1/100 patients (only those not already listed in the tabulated results from placebo-controlled trials appear in this listing); those occurring in 1/100 to 1/1000 patients; and those occurring in fewer than 1/1000 patients.
Blood and lymphatic disorders: <1/1000 patients: thrombocytopenia; ≥1/1000 patients and <1/100 patients: anemia
Cardiac disorders: ≥1/1000 patients and <1/100 patients: tachycardia, temporary bundle branch block
Eye disorders: ≥1/1000 patients and <1/100 patients: accommodation disorder
Gastrointestinal disorders: ≥1/1000 patients and <1/100 patients: oral paraesthesia, glossodynia, swollen tongue
General disorders: <1/1000 patients: idiosyncratic drug reaction
Investigations: ≥1/1000 patients and <1/100 patients: hyponatremia
Nervous system disorders: ≥1/1000 patients and <1/100 patients: dysarthria
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of SAPHRIS. Application site reactions, primarily in the sublingual area, have been reported. These application site reactions included oral ulcers, blisters, peeling/sloughing, and inflammation. In many cases, the occurrence of these application site reactions led to discontinuation of therapy.
7 DRUG INTERACTIONS
The risks of using SAPHRIS in combination with other drugs have not been extensively evaluated. Given the primary CNS effects of SAPHRIS, caution should be used when it is taken in combination with other centrally acting drugs or alcohol.
Because of its α1-adrenergic antagonism with potential for inducing hypotension, SAPHRIS may enhance the effects of certain antihypertensive agents.
7.1 Potential for Other Drugs to Affect SAPHRIS
Asenapine is cleared primarily through direct glucuronidation by UGT1A4 and oxidative metabolism by cytochrome P450 isoenzymes (predominantly CYP1A2). The potential effects of inhibitors of several of these enzyme pathways on asenapine clearance were studied.
| Coadministered drug (Postulated effect on CYP450/UGT) | Dose schedules | Effect on asenapine pharmacokinetics | Recommendation | ||
|---|---|---|---|---|---|
| Coadministered drug | Asenapine | Cmax | AUC0-∞ | ||
|
|||||
| Fluvoxamine (CYP1A2 inhibitor) | 25 mg twice daily for 8 days | 5-mg Single Dose | +13% | +29% | Coadminister with caution* |
| Paroxetine (CYP2D6 inhibitor) | 20 mg once daily for 9 days | 5-mg Single Dose | –13% | –9% | No SAPHRIS dose adjustment required [see Drug Interactions (7.2)] |
| Imipramine (CYP1A2/2C19/3A4 inhibitor) | 75-mg Single Dose | 5-mg Single Dose | +17% | +10% | No SAPHRIS dose adjustment required |
| Cimetidine (CYP3A4/2D6/1A2 inhibitor) | 800 mg twice daily for 8 days | 5-mg Single Dose | –13% | +1% | No SAPHRIS dose adjustment required |
| Carbamazepine (CYP3A4 inducer) | 400 mg twice daily for 15 days | 5-mg Single Dose | –16% | –16% | No SAPHRIS dose adjustment required |
| Valproate (UGT1A4 inhibitor) | 500 mg twice daily for 9 days | 5-mg Single Dose | 2% | –1% | No SAPHRIS dose adjustment required |
A population pharmacokinetic analysis indicated that the concomitant administration of lithium had no effect on the pharmacokinetics of asenapine.
7.2 Potential for SAPHRIS to Affect Other Drugs
Coadministration with CYP2D6 Substrates: In vitro studies indicate that asenapine weakly inhibits CYP2D6.
Following coadministration of dextromethorphan and SAPHRIS in healthy subjects, the ratio of dextrorphan/dextromethorphan (DX/DM) as a marker of CYP2D6 activity was measured. Indicative of CYP2D6 inhibition, treatment with SAPHRIS 5 mg twice daily decreased the DX/DM ratio to 0.43. In the same study, treatment with paroxetine 20 mg daily decreased the DX/DM ratio to 0.032. In a separate study, coadministration of a single 75-mg dose of imipramine with a single 5-mg dose of SAPHRIS did not affect the plasma concentrations of the metabolite desipramine (a CYP2D6 substrate). Thus, in vivo, SAPHRIS appears to be at most a weak inhibitor of CYP2D6. Coadministration of a single 20-mg dose of paroxetine (a CYP2D6 substrate and inhibitor) during treatment with 5 mg SAPHRIS twice daily in 15 healthy male subjects resulted in an almost 2-fold increase in paroxetine exposure. Asenapine may enhance the inhibitory effects of paroxetine on its own metabolism.
SAPHRIS should be coadministered cautiously with drugs that are both substrates and inhibitors for CYP2D6.
Valproic acid and lithium pre-dose serum concentrations collected from an adjunctive therapy study were comparable between asenapine treated patients and placebo treated patients indicating a lack of effect of asenapine on valproic and lithium plasma levels.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category C: There are no adequate and well-controlled studies of SAPHRIS in pregnant women.
In animal studies, asenapine increased post-implantation loss and decreased pup weight and survival at doses similar to or less than recommended clinical doses. In these studies there was no increase in the incidence of structural abnormalities caused by asenapine.
Asenapine was not teratogenic in reproduction studies in rats and rabbits at intravenous doses up to 1.5 mg/kg in rats and 0.44 mg/kg in rabbits. These doses are 0.7 and 0.4 times, respectively, the maximum recommended human dose (MRHD) of 10 mg twice daily given sublingually on a mg/m2 basis. Plasma levels of asenapine were measured in the rabbit study, and the area under the curve (AUC) at the highest dose tested was 2 times that in humans receiving the MRHD.
In a study in which rats were treated from day 6 of gestation through day 21 postpartum with intravenous doses of asenapine of 0.3, 0.9, and 1.5 mg/kg/day (0.15, 0.4, and 0.7 times the MRHD of 10 mg twice daily given sublingually on a mg/m2 basis), increases in post-implantation loss and early pup deaths were seen at all doses, and decreases in subsequent pup survival and weight gain were seen at the two higher doses. A cross-fostering study indicated that the decreases in pup survival were largely due to prenatal drug effects. Increases in post-implantation loss and decreases in pup weight and survival were also seen when pregnant rats were dosed orally with asenapine.
Non-teratogenic Effects
Neonates exposed to antipsychotic drugs during the third trimester of pregnancy are at risk for extrapyramidal and/or withdrawal symptoms following delivery. There have been reports of agitation, hypertonia, hypotonia, tremor, somnolence, respiratory distress and feeding disorder in these neonates. These complications have varied in severity; while in some cases symptoms have been self-limited, in other cases neonates have required intensive care unit support and prolonged hospitalization. SAPHRIS (asenapine) should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
8.3 Nursing Mothers
Asenapine is excreted in milk of rats during lactation. It is not known whether asenapine or its metabolites are excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when SAPHRIS is administered to a nursing woman. It is recommended that women receiving SAPHRIS should not breast feed.
8.5 Geriatric Use
Clinical studies of SAPHRIS in the treatment of schizophrenia and bipolar mania did not include sufficient numbers of patients aged 65 and over to determine whether or not they respond differently than younger patients. Of the approximately 2250 patients in pre-marketing clinical studies of SAPHRIS, 1.1% (25) were 65 years of age or over. Multiple factors that might increase the pharmacodynamic response to SAPHRIS, causing poorer tolerance or orthostasis, could be present in elderly patients, and these patients should be monitored carefully.
In elderly patients with psychosis, asenapine exposure (AUC) was on average 40% higher compared to younger adult patients [see Clinical Pharmacology (12.3)].
Elderly patients with dementia-related psychosis treated with SAPHRIS are at an increased risk of death compared to placebo. SAPHRIS is not approved for the treatment of patients with dementia-related psychosis [see Boxed Warning].
8.6 Renal Impairment
The exposure of asenapine following a single dose of 5 mg was similar among subjects with varying degrees of renal impairment and subjects with normal renal function [see Clinical Pharmacology (12.3)].
8.7 Hepatic Impairment
In subjects with severe hepatic impairment who were treated with a single dose of SAPHRIS 5 mg, asenapine exposures (on average), were 7-fold higher than the exposures observed in subjects with normal hepatic function. Thus, SAPHRIS is not recommended in patients with severe hepatic impairment (Child-Pugh C) [see Dosage and Administration (2.4) and Clinical Pharmacology (12.3)].
9 DRUG ABUSE AND DEPENDENCE
9.2 Abuse
SAPHRIS has not been systematically studied in animals or humans for its abuse potential or its ability to induce tolerance or physical dependence. Thus, it is not possible to predict the extent to which a CNS-active drug will be misused, diverted and/or abused once it is marketed. Patients should be evaluated carefully for a history of drug abuse, and such patients should be observed carefully for signs that they are misusing or abusing SAPHRIS (e.g., drug-seeking behavior, increases in dose).
10 OVERDOSAGE
Human Experience: In pre-marketing clinical studies involving more than 3350 patients and/or healthy subjects, accidental or intentional acute overdosage of SAPHRIS was identified in 3 patients. Among these few reported cases of overdose, the highest estimated ingestion of SAPHRIS was 400 mg. Reported adverse reactions at the highest dosage included agitation and confusion.
Management of Overdosage: There is no specific antidote to SAPHRIS. The possibility of multiple drug involvement should be considered. An electrocardiogram should be obtained and management of overdose should concentrate on supportive therapy, maintaining an adequate airway, oxygenation and ventilation, and management of symptoms.
Hypotension and circulatory collapse should be treated with appropriate measures, such as intravenous fluids and/or sympathomimetic agents (epinephrine and dopamine should not be used, since beta stimulation may worsen hypotension in the setting of SAPHRIS-induced alpha blockade). In case of severe extrapyramidal symptoms, anticholinergic medication should be administered. Close medical supervision and monitoring should continue until the patient recovers.
11 DESCRIPTION
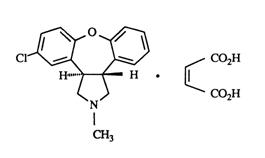
SAPHRIS is a psychotropic agent that is available for sublingual administration. Asenapine belongs to the class dibenzo-oxepino pyrroles. The chemical designation is (3aRS,12bRS)-5-Chloro-2-methyl-2,3,3a,12b-tetrahydro-1Hdibenzo[2,3:6,7]oxepino[4,5-c]pyrrole (2Z)-2-butenedioate (1:1). Its molecular formula is C17H16ClNO•C4H4O4 and its molecular weight is 401.84 (free base: 285.8). The chemical structure is:
Asenapine is a white to off-white powder.
SAPHRIS is supplied for sublingual administration in tablets containing 5-mg or 10-mg asenapine; inactive ingredients include gelatin and mannitol.
SAPHRIS, black cherry flavor, is supplied for sublingual administration in tablets containing 5-mg or 10-mg asenapine; inactive ingredients include gelatin, mannitol, sucralose, and black cherry flavor.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The mechanism of action of asenapine, as with other drugs having efficacy in schizophrenia and bipolar disorder, is unknown. It has been suggested that the efficacy of asenapine in schizophrenia is mediated through a combination of antagonist activity at D2 and 5-HT2A receptors.
12.2 Pharmacodynamics
Asenapine exhibits high affinity for serotonin 5-HT1A, 5-HT1B, 5-HT2A, 5-HT2B, 5-HT2C, 5-HT5, 5-HT6, and 5-HT7 receptors (Ki values of 2.5, 4.0, 0.06, 0.16, 0.03, 1.6, 0.25, and 0.13 nM), dopamine D2, D3, D4, and D1 receptors (Ki values of 1.3, 0.42, 1.1, and 1.4 nM), α1 and α2-adrenergic receptors (Ki values of 1.2 and 1.2 nM), and histamine H1 receptors (Ki value 1.0 nM), and moderate affinity for H2 receptors (Ki value of 6.2 nM). In in vitro assays asenapine acts as an antagonist at these receptors. Asenapine has no appreciable affinity for muscarinic cholinergic receptors (e.g., Ki value of 8128 nM for M1).
12.3 Pharmacokinetics
Following a single 5-mg dose of SAPHRIS, the mean Cmax was approximately 4 ng/mL and was observed at a mean tmax of 1 hour. Elimination of asenapine is primarily through direct glucuronidation by UGT1A4 and oxidative metabolism by cytochrome P450 isoenzymes (predominantly CYP1A2). Following an initial more rapid distribution phase, the mean terminal half-life is approximately 24 hrs. With multiple-dose twice-daily dosing, steady-state is attained within 3 days. Overall, steady-state asenapine pharmacokinetics are similar to single-dose pharmacokinetics.
Absorption: Following sublingual administration, asenapine is rapidly absorbed with peak plasma concentrations occurring within 0.5 to 1.5 hours. The absolute bioavailability of sublingual asenapine at 5 mg is 35%. Increasing the dose from 5 mg to 10 mg twice daily (a two-fold increase) results in less than linear (1.7 times) increases in both the extent of exposure and maximum concentration. The absolute bioavailability of asenapine when swallowed is low (<2% with an oral tablet formulation).
The intake of water several (2 or 5) minutes after asenapine administration resulted in decreased asenapine exposure. Therefore, eating and drinking should be avoided for 10 minutes after administration [see Dosage and Administration (2.1)].
Distribution: Asenapine is rapidly distributed and has a large volume of distribution (approximately 20 - 25 L/kg), indicating extensive extravascular distribution. Asenapine is highly bound (95%) to plasma proteins, including albumin and α1-acid glycoprotein.
Metabolism and Elimination: Direct glucuronidation by UGT1A4 and oxidative metabolism by cytochrome P450 isoenzymes (predominantly CYP1A2) are the primary metabolic pathways for asenapine.
Asenapine is a high clearance drug with a clearance after intravenous administration of 52 L/h. In this circumstance, hepatic clearance is influenced primarily by changes in liver blood flow rather than by changes in the intrinsic clearance, i.e., the metabolizing enzymatic activity. Following an initial more rapid distribution phase, the terminal half life of asenapine is approximately 24 hours. Steady-state concentrations of asenapine are reached within 3 days of twice daily dosing.
After administration of a single dose of [14C]-labeled asenapine, about 90% of the dose was recovered; approximately 50% was recovered in urine, and 40% recovered in feces. About 50% of the circulating species in plasma have been identified. The predominant species was asenapine N+-glucuronide; others included N-desmethylasenapine, N-desmethylasenapine N-carbamoyl glucuronide, and unchanged asenapine in smaller amounts. SAPHRIS activity is primarily due to the parent drug.
In vitro studies indicate that asenapine is a substrate for UGT1A4, CYP1A2 and to a lesser extent CYP3A4 and CYP2D6. Asenapine is a weak inhibitor of CYP2D6. Asenapine does not cause induction of CYP1A2 or CYP3A4 activities in cultured human hepatocytes. Coadministration of asenapine with known inhibitors, inducers or substrates of these metabolic pathways has been studied in a number of drug-drug interaction studies [see Drug Interactions (7)].
Smoking: A population pharmacokinetic analysis indicated that smoking, which induces CYP1A2, had no effect on the clearance of asenapine in smokers. In a crossover study in which 24 healthy male subjects (who were smokers) were administered a single 5-mg sublingual dose, concomitant smoking had no effect on the pharmacokinetics of asenapine.
Food: A crossover study in 26 healthy male subjects was performed to evaluate the effect of food on the pharmacokinetics of a single 5-mg dose of asenapine. Consumption of food immediately prior to sublingual administration decreased asenapine exposure by 20%; consumption of food 4 hours after sublingual administration decreased asenapine exposure by about 10%. These effects are probably due to increased hepatic blood flow.
In clinical trials establishing the efficacy and safety of SAPHRIS, patients were instructed to avoid eating for 10 minutes following sublingual dosing. There were no other restrictions with regard to the timing of meals in these trials [see Dosage and Administration (2.1) and Patient Counseling Information (17.1)].
Water: In clinical trials establishing the efficacy and safety of SAPHRIS, patients were instructed to avoid drinking for 10 minutes following sublingual dosing. The effect of water administration following 10-mg sublingual SAPHRIS dosing was studied at different time points of 2, 5, 10, and 30 minutes in 15 healthy male subjects. The exposure of asenapine following administration of water 10 minutes after sublingual dosing was equivalent to that when water was administered 30 minutes after dosing. Reduced exposure to asenapine was observed following water administration at 2 minutes (19% decrease) and 5 minutes (10% decrease) [see Dosage and Administration (2.1) and Patient Counseling Information (17.1)].
Special Populations:
Hepatic Impairment: The effect of decreased hepatic function on the pharmacokinetics of asenapine, administered as a single 5-mg sublingual dose, was studied in 30 subjects (8 each in those with normal hepatic function and Child-Pugh A and B groups, and 6 in the Child-Pugh C group). In subjects with mild or moderate hepatic impairment (Child-Pugh A or B), asenapine exposure was 12% higher than that in subjects with normal hepatic function, indicating that dosage adjustment is not required for these subjects. In subjects with severe hepatic impairment, asenapine exposures were on average 7 times higher than the exposures of those in subjects with normal hepatic function. Thus, SAPHRIS is not recommended in patients with severe hepatic impairment (Child-Pugh C) [see Dosage in Specific Populations (2.4) and Use in Specific Populations (8.7) and Warnings and Precautions (5.14)].
Renal Impairment: The effect of decreased renal function on the pharmacokinetics of asenapine was studied in subjects with mildly (creatinine clearance (CrCl) 51 to 80 mL/min; N=8), moderately (CrCl 30 to 50 mL/min; N=8), and severely (CrCl less than 30 mL/min but not on dialysis; N=8) impaired renal function and compared to normal subjects (CrCl greater than 80 mL/min; N=8). The exposure of asenapine following a single dose of 5 mg was similar among subjects with varying degrees of renal impairment and subjects with normal renal function. Dosage adjustment based upon degree of renal impairment is not required. The effect of renal function on the excretion of other metabolites and the effect of dialysis on the pharmacokinetics of asenapine has not been studied [see Use in Specific Populations (8.6)].
Geriatric Patients: In elderly patients (N=96) with psychosis (65-85 years of age), asenapine exposure (AUC) was on average 40% higher compared to younger adult patients. No dosage adjustment is necessary. In a population pharmacokinetic analysis, a decrease in clearance with increasing age was observed, implying a 30% higher exposure in elderly as compared to adult patients [see Use in Specific Populations (8.5)].
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis: In a lifetime carcinogenicity study in CD-1 mice asenapine was administered subcutaneously at doses up to those resulting in plasma levels (AUC) estimated to be 5 times those in humans receiving the MRHD of 10 mg twice daily. The incidence of malignant lymphomas was increased in female mice, with a no-effect dose resulting in plasma levels estimated to be 1.5 times those in humans receiving the MRHD. The mouse strain used has a high and variable incidence of malignant lymphomas, and the significance of these results to humans is unknown. There were no increases in other tumor types in female mice. In male mice, there were no increases in any tumor type.
In a lifetime carcinogenicity study in Sprague-Dawley rats, asenapine did not cause any increases in tumors when administered subcutaneously at doses up to those resulting in plasma levels (AUC) estimated to be 5 times those in humans receiving the MRHD.
Mutagenesis: No evidence for genotoxic potential of asenapine was found in the in vitro bacterial reverse mutation assay, the in vitro forward gene mutation assay in mouse lymphoma cells, the in vitro chromosomal aberration assays in human lymphocytes, the in vitro sister chromatid exchange assay in rabbit lymphocytes, or the in vivo micronucleus assay in rats.
14 CLINICAL STUDIES
14.1 Schizophrenia
The efficacy of SAPHRIS in the treatment of schizophrenia in adults was evaluated in three fixed-dose, short-term (6 week), randomized, double-blind, placebo-controlled, and active-controlled (haloperidol, risperidone, and olanzapine) trials of adult patients who met DSM-IV criteria for schizophrenia and were having an acute exacerbation of their schizophrenic illness. In two of the three trials SAPHRIS demonstrated superior efficacy to placebo. In a third trial, SAPHRIS could not be distinguished from placebo; however, an active control in that trial was superior to placebo.
In the two positive trials for SAPHRIS, the primary efficacy rating scale was the Positive and Negative Syndrome Scale (PANSS), which assesses the symptoms of schizophrenia. The primary endpoint was change from baseline to endpoint on the PANSS total score. The results of the SAPHRIS trials in schizophrenia follow:
In trial 1, a 6-week trial (n=174), comparing SAPHRIS (5 mg twice daily) to placebo, SAPHRIS 5 mg twice daily was statistically superior to placebo on the PANSS total score.
In trial 2, a 6-week trial (n=448), comparing two fixed doses of SAPHRIS (5 mg and 10 mg twice daily) to placebo, SAPHRIS 5 mg twice daily was statistically superior to placebo on the PANSS total score. SAPHRIS 10 mg twice daily showed no added benefit compared to 5 mg twice daily and was not significantly different from placebo.
An examination of population subgroups did not reveal any clear evidence of differential responsiveness on the basis of age, gender or race.
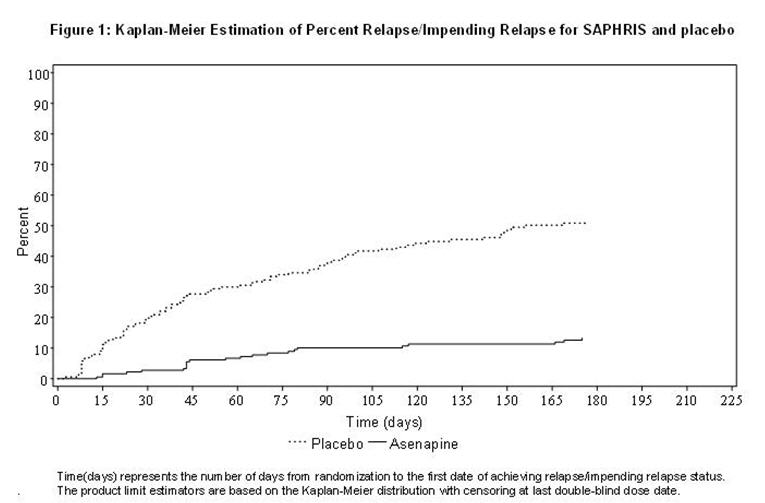
Maintenance of efficacy has been demonstrated in a placebo-controlled, double-blind, multicenter, flexible dose (5 mg or 10 mg twice daily based on tolerability) clinical trial with a randomized withdrawal design. A total of 700 patients entered open-label treatment with SAPHRIS for a period of 26 weeks. Of these, a total of 386 patients who met pre-specified criteria for continued stability (mean length of stabilization was 22 weeks) were randomized to a double-blind, placebo-controlled, randomized withdrawal phase. SAPHRIS was statistically superior to placebo in time to relapse or impending relapse defined as increase in PANSS ≥20% from baseline and a Clinical Global Impression–Severity of Illness (CGI-S) score ≥4 (at least 2 days within 1 week) or PANSS score ≥5 on "hostility" or "uncooperativeness" items and CGI-S score ≥4 (≥2 days within a week), or PANSS score ≥5 on any two of the following items: "unusual thought content," "conceptual disorganization," or "hallucinatory behavior" items, and CGI-S score ≥4 (≥2 days within 1 week) or investigator judgment of worsening symptoms or increased risk of violence to self (including suicide) or other persons. The Kaplan-Meier curves of the time to relapse or impending relapse during the double-blind, placebo-controlled, randomized withdrawal phase of this trial for SAPHRIS and placebo are shown in Figure 1.
14.2 Bipolar Disorder
Monotherapy: The efficacy of SAPHRIS in the treatment of acute mania was established in two similarly designed 3-week, randomized, double-blind, placebo-controlled, and active-controlled (olanzapine) trials of adult patients who met DSM-IV criteria for Bipolar I Disorder with an acute manic or mixed episode with or without psychotic features.
The primary rating instrument used for assessing manic symptoms in these trials was the Young Mania Rating Scale (YMRS). Patients were also assessed on the Clinical Global Impression – Bipolar (CGI-BP) scale. In both trials, all patients randomized to SAPHRIS were initially administered 10 mg twice daily, and the dose could be adjusted within the dose range of 5 to 10 mg twice daily from Day 2 onward based on efficacy and tolerability. Ninety percent of patients remained on the 10-mg twice daily dose. SAPHRIS was statistically superior to placebo on the YMRS total score and the CGI-BP Severity of Illness score (mania) in both studies.
An examination of subgroups did not reveal any clear evidence of differential responsiveness on the basis of age, gender or race.
Adjunctive Therapy: The efficacy of SAPHRIS as an adjunctive therapy in acute mania was established in a 12-week, placebo-controlled trial with a 3-week primary efficacy endpoint involving 326 patients with a manic or mixed episode of Bipolar I Disorder, with or without psychotic features, who were partially responsive to lithium or valproate monotherapy after at least 2 weeks of treatment. SAPHRIS was statistically superior to placebo in the reduction of manic symptoms (measured by the YMRS total score) as an adjunctive therapy to lithium or valproate monotherapy at week 3.
16 HOW SUPPLIED/STORAGE AND HANDLING
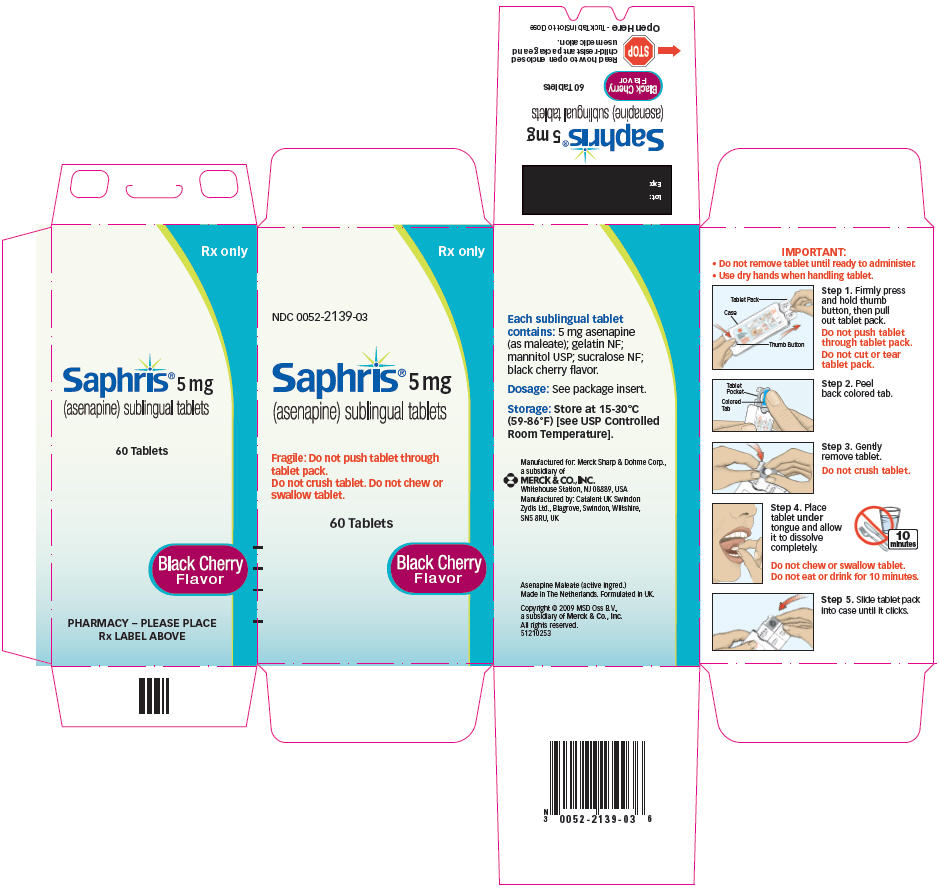
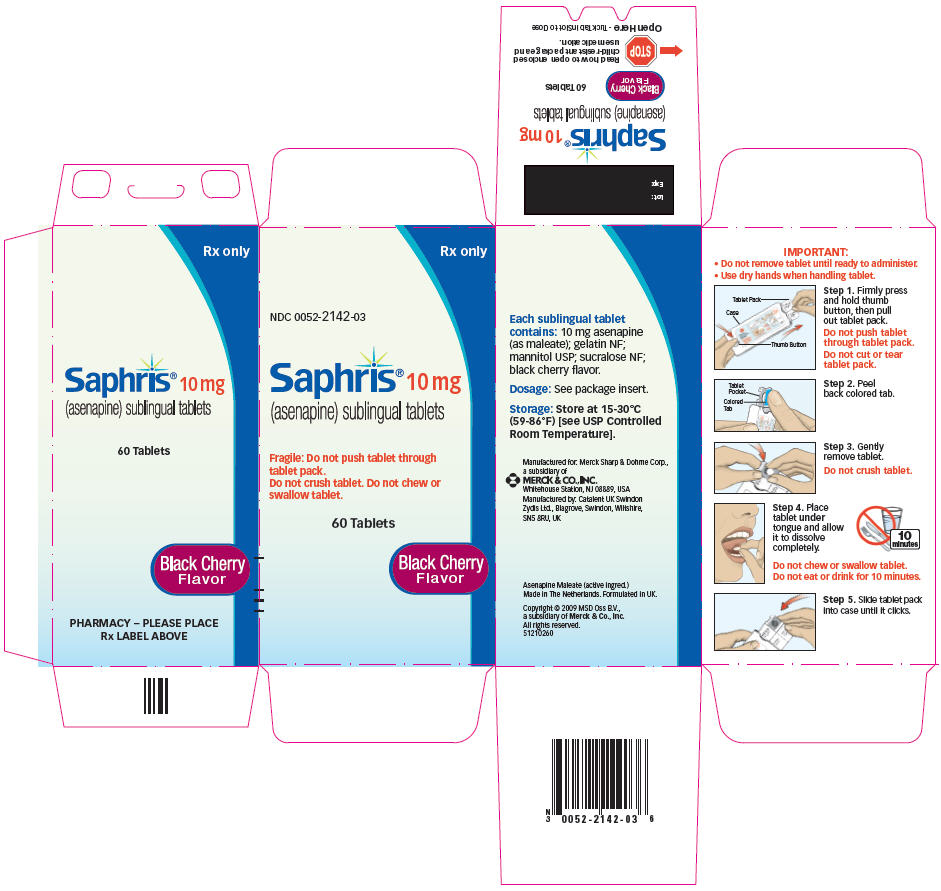
SAPHRIS (asenapine) sublingual tablets are supplied as:
5-mg Tablets
Round, white to off-white sublingual tablets, with "5" on one side.
- Child-resistant packaging
Box of 60 6 blisters with 10 tablets NDC 0052-0118-06 - Hospital Unit Dose
Box of 100 10 blisters with 10 tablets NDC 0052-0118-90
10-mg Tablets
Round, white to off-white sublingual tablets, with "10" on one side.
- Child-resistant packaging
Box of 60 6 blisters with 10 tablets NDC 0052-0119-06 - Hospital Unit Dose
Box of 100 10 blisters with 10 tablets NDC 0052-0119-90
5-mg Tablets, black cherry flavor
Round, white to off-white sublingual tablets, with "5" on one side within a circle.
- Child-resistant packaging
Box of 60 6 blisters with 10 tablets NDC 0052-2139-03
- Hospital Unit Dose
Box of 100 10 blisters with 10 tablets NDC 0052-2139-04
10-mg Tablets, black cherry flavor
Round, white to off-white sublingual tablets, with "10" on one side within a circle.
- Child-resistant packaging
Box of 60 6 blisters with 10 tablets NDC 0052-2142-03
- Hospital Unit Dose
Box of 100 10 blisters with 10 tablets NDC 0052-2142-04
17 PATIENT COUNSELING INFORMATION
17.1 Tablet Administration
IMPORTANT:
- Do not remove tablet until ready to administer.
-
Use dry hands when handling tablet.
-
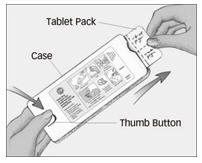
Step 1. Firmly press and hold thumb button, then pull out tablet pack.
Do not push tablet through tablet pack.
Do not cut or tear tablet pack.
-
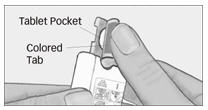
Step 2. Peel back colored tab.
-
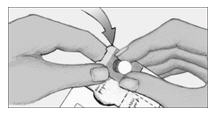
Step 3. Gently remove tablet.
Do not crush tablet.
-
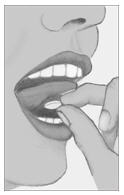
Step 4. Place tablet under tongue and allow it to dissolve completely.
-
Do not chew or swallow tablet.
Do not eat or drink for 10 minutes.
- Step 5. Slide tablet pack into case until it clicks.
[see Drug Interactions (7) and Clinical Pharmacology (12.3)].
17.2 Increased Mortality in Elderly Patients with Dementia-Related Psychosis
Patients and caregivers should be advised that elderly patients with dementia-related psychoses treated with atypical antipsychotic drugs are at increased risk of death compared with placebo. SAPHRIS is not approved for elderly patients with dementia-related psychosis [see Warnings and Precautions (5.1)].
17.3 Hypersensitivity Reactions
Patients should be informed of the signs and symptoms of a serious allergic reaction (e.g., difficulty breathing, itching, swelling of the face, tongue or throat, feeling lightheaded etc.). Patients should be instructed to seek immediate emergency assistance if they develop any of these signs and symptoms [see Warnings and Precautions (5.7) and Adverse Reactions (6)].
17.4 Application Site Reactions
Inform patients that application site reactions including oral ulcers, blisters, peeling/sloughing and inflammation have been reported. Instruct patients to monitor for these reactions [see Adverse Reactions (6.2)].
17.5 Neuroleptic Malignant Syndrome
Patients and caregivers should be counseled that a potentially fatal symptom complex sometimes referred to as Neuroleptic Malignant Syndrome (NMS) has been reported in association with administration of antipsychotic drugs. Signs and symptoms of NMS include hyperpyrexia, muscle rigidity, altered mental status, and evidence of autonomic instability (irregular pulse or blood pressure, tachycardia, diaphoresis, and cardiac dysrhythmia) [see Warnings and Precautions (5.3)].
17.6 Hyperglycemia and Diabetes Mellitus
Patients should be aware of the symptoms of hyperglycemia (high blood sugar) and diabetes mellitus. Patients who are diagnosed with diabetes, those with risk factors for diabetes, or those that develop these symptoms during treatment should have their blood glucose monitored at the beginning of and periodically during treatment [see Warnings and Precautions (5.5)].
17.7 Weight Gain
Patients should be advised that they may experience weight gain. Patients should have their weight monitored regularly [see Warnings and Precautions (5.6)].
17.8 Orthostatic Hypotension
Patients should be advised of the risk of orthostatic hypotension (symptoms include feeling dizzy or lightheaded upon standing) especially early in treatment, and also at times of re-initiating treatment or increases in dose [see Warnings and Precautions (5.8)].
17.9 Leukopenia/Neutropenia
Patients with a pre-existing low WBC or a history of drug induced leukopenia/neutropenia should be advised that they should have their CBC monitored while taking SAPHRIS [see Warnings and Precautions (5.9)].
17.10 Interference with Cognitive and Motor Performance
Patients should be cautioned about performing activities requiring mental alertness, such as operating hazardous machinery or operating a motor vehicle, until they are reasonably certain that SAPHRIS therapy does not affect them adversely [see Warnings and Precautions (5.13)].
17.11 Heat Exposure and Dehydration
Patients should be advised regarding appropriate care in avoiding overheating and dehydration [see Warnings and Precautions (5.14)].
17.12 Concomitant Medication and Alcohol
Patients should be advised to inform their physicians if they are taking, or plan to take, any prescription or over-the-counter medications since there is a potential for interactions. Patients should be advised to avoid alcohol while taking SAPHRIS [see Drug Interactions (7)].
17.13 Pregnancy and Nursing
Patients should be advised to notify their physician if they become pregnant or intend to become pregnant during therapy with SAPHRIS. Patients should be advised not to breast feed if they are taking SAPHRIS [see Use in Special Populations (8.1, 8.3)].
Manufactured for: Merck Sharp & Dohme Corp., a subsidiary of
MERCK & CO., INC., Whitehouse Station, NJ 08889, USA
Manufactured by: Catalent UK Swindon Zydis Ltd., Blagrove, Swindon, Wiltshire, SN5 8RU, UK
U.S. Patent Nos. 5,763,476 and 7,741,358.
Copyright © 2009, 2011 MSD Oss B.V., a subsidiary of Merck & Co., Inc.
All rights reserved.
Revised: 03/2013
900274-ASP-TB-USPI.25
PRINCIPAL DISPLAY PANEL - 5 mg Bottle Carton
Rx only
NDC 0052-0118-06
Saphris® 5 mg
(asenapine) sublingual tablets
Fragile: Do not push tablet through
tablet pack.
Do not crush tablet. Do not chew or
swallow tablet.
60 Tablets
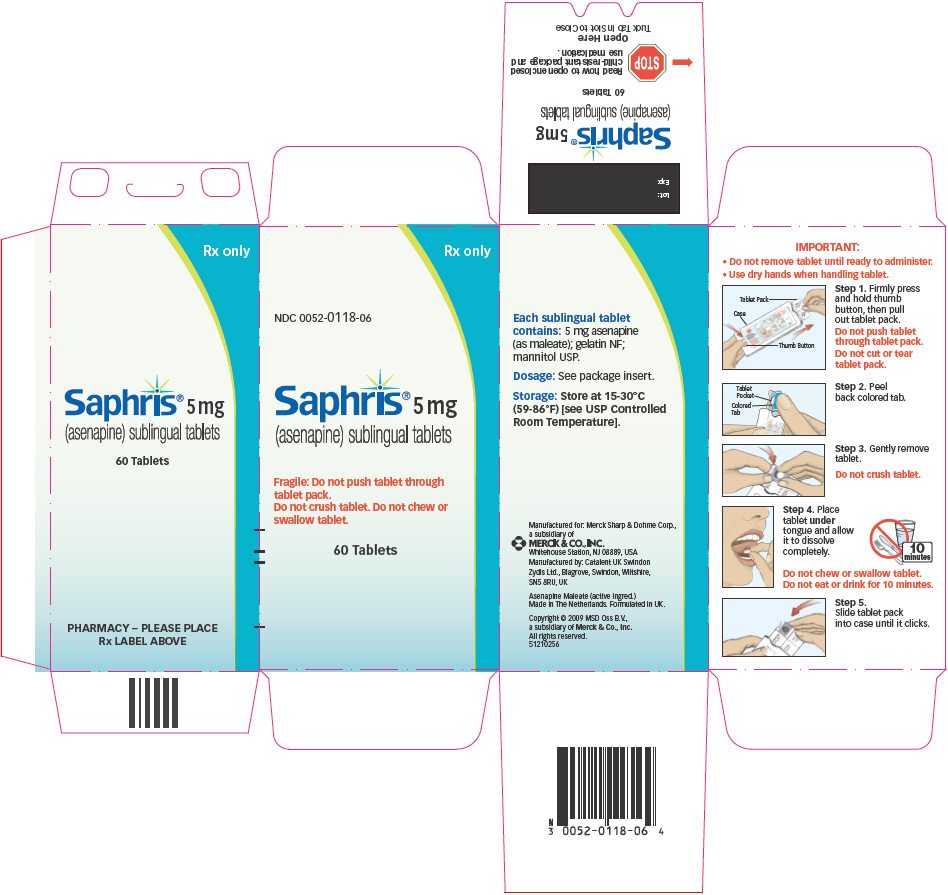
PRINCIPAL DISPLAY PANEL - 10 mg Bottle Carton
Rx only
NDC 0052-0119-06
Saphris® 10 mg
(asenapine) sublingual tablets
Fragile: Do not push tablet through
tablet pack.
Do not crush tablet. Do not chew or
swallow tablet.
60 Tablets
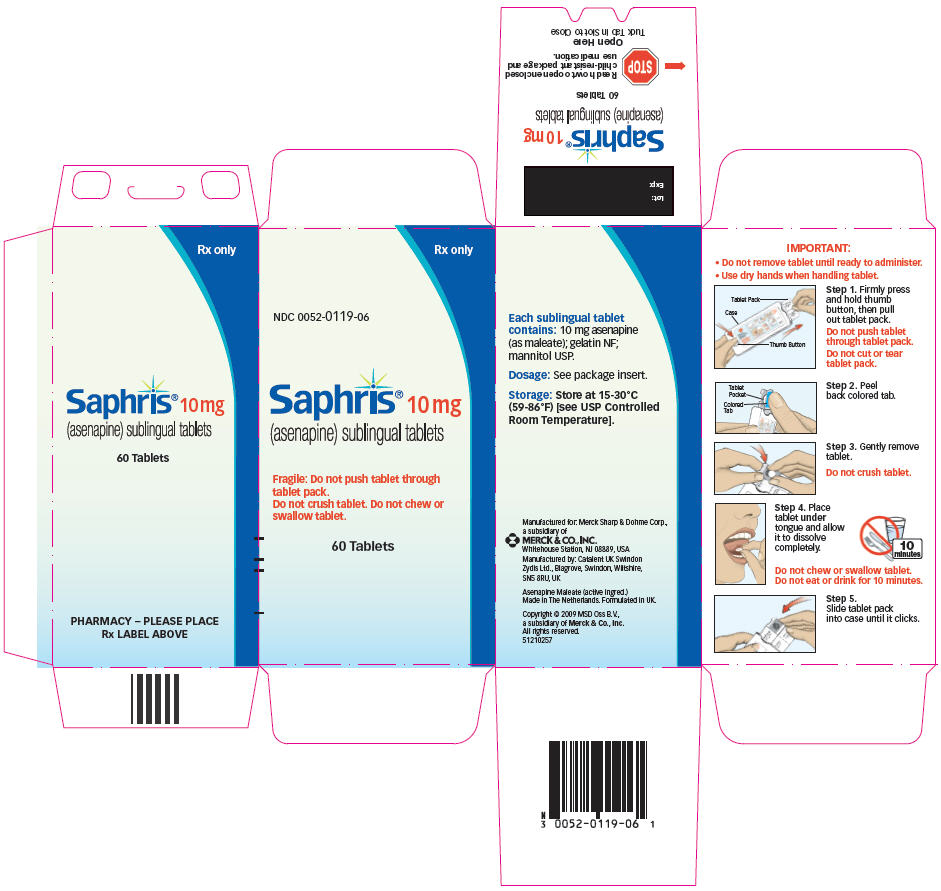
| SAPHRIS
asenapine maleate tablet |
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
| SAPHRIS
asenapine maleate tablet |
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
| SAPHRIS
asenapine maleate tablet |
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
| SAPHRIS
asenapine maleate tablet |
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Merck Sharp & Dohme Corp (078796541) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Catalent Pharma Solutions Limited | 237676320 | MANUFACTURE(0052-0118, 0052-0119, 0052-2139, 0052-2142) | |