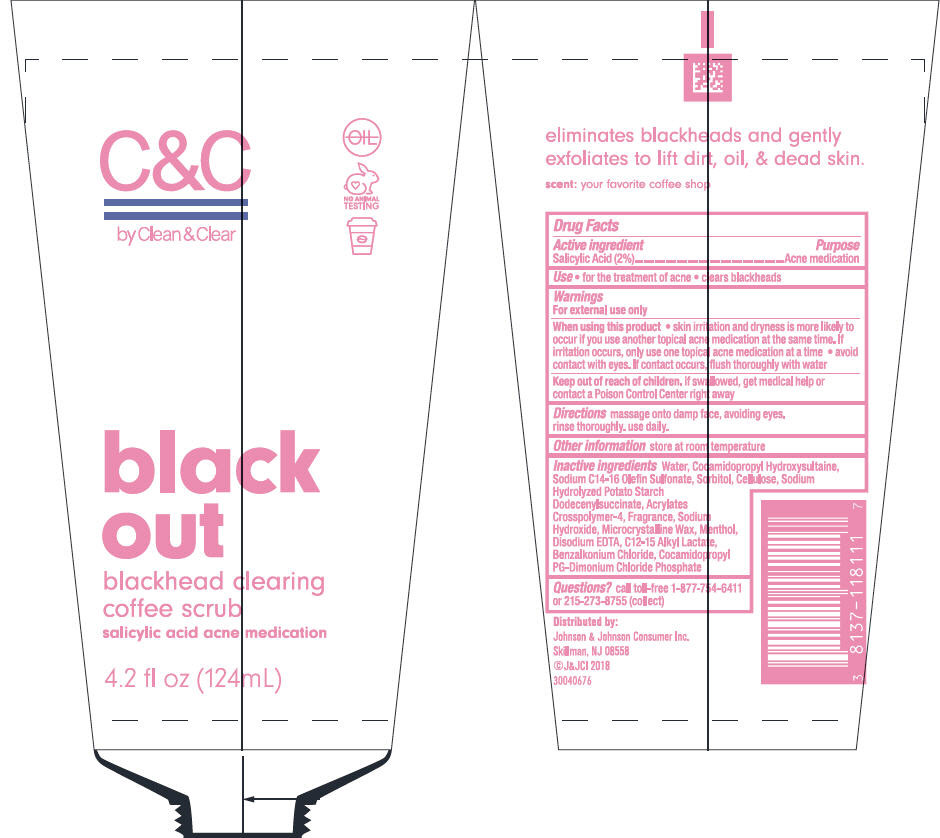
C AND C BY CLEAN AND CLEAR BLACK OUT BLACKHEAD CLEARING COFFEE SCRUB- salicylic acid lotion
Johnson & Johnson Consumer Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
C & C by Clean & Clear black out blackhead clearing coffee scrub
Warnings
For external use only
Inactive ingredients
Water, Cocamidopropyl Hydroxysultaine, Sodium C14-16 Olefin Sulfonate, Sorbitol, Cellulose, Sodium Hydrolyzed Potato Starch Dodecenylsuccinate, Acrylates Crosspolymer-4, Fragrance, Sodium Hydroxide, Microcrystalline Wax, Menthol, Disodium EDTA, C12-15 Alkyl Lactate, Benzalkonium Chloride, Cocamidopropyl PG-Dimonium Chloride Phosphate
| C AND C BY CLEAN AND CLEAR BLACK OUT BLACKHEAD CLEARING COFFEE SCRUB
salicylic acid lotion |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| Labeler - Johnson & Johnson Consumer Inc. (002347102) |
Revised: 6/2020
Document Id: d908003f-a5ca-49f7-b50e-f4da415c160a
Set id: 16f5981f-379c-4446-8157-40623f47a090
Version: 2
Effective Time: 20200624
Johnson & Johnson Consumer Inc.