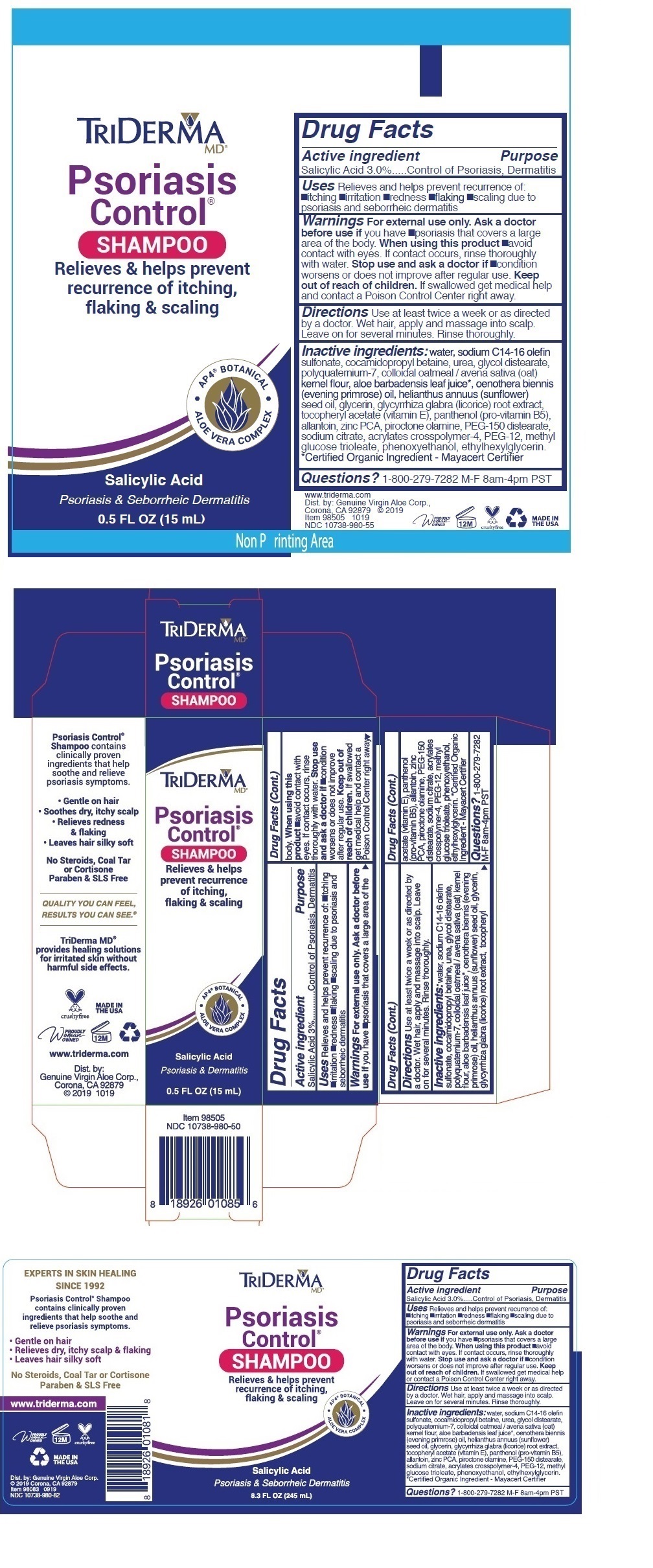
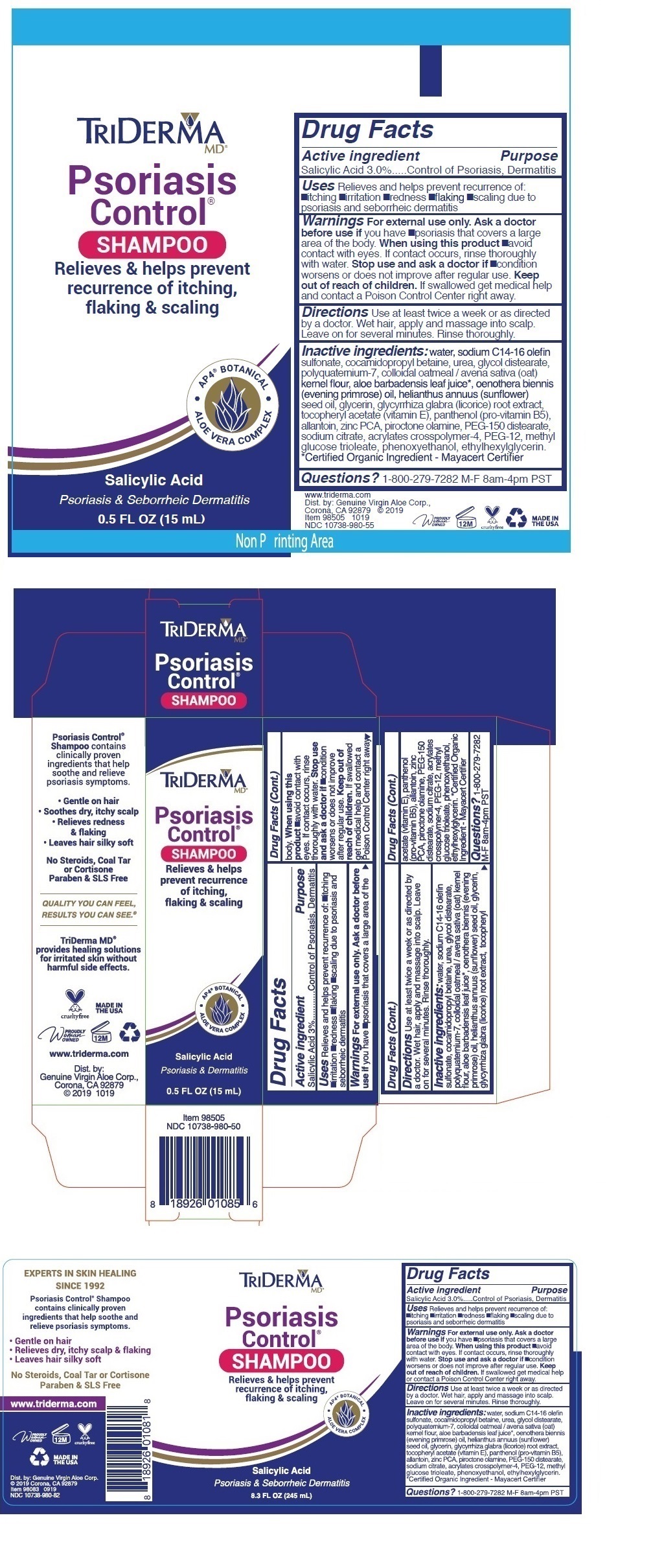
Label: TRIDERMA PSORIASIS CONTROL- salicylic acid shampoo
- NDC Code(s): 10738-980-50, 10738-980-55, 10738-980-82
- Packager: Genuine Virgin Aloe Corporation
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated February 2, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredient
- Purpose
- INDICATIONS & USAGE
-
WARNINGS
Warnings For external use only. Ask a doctor before use if you have •psoriasis that covers a large area of the body. When using this product •avoid contact with eyes. If contact occurs, rinse thoroughly with water. Stop use and ask a doctor if •condition worsens or does not improve after regular use.
- DOSAGE & ADMINISTRATION
-
INACTIVE INGREDIENT
Inactive ingredients: water, sodium C14-16 olefin sulfonate, cocamidopropyl betaine, urea, glycol distearate, polyquaternium-7, colloidal oatmeal / avena sativa (oat) kernel flour, aloe barbadensis leaf juice*, oenothera biennis (evening primrose) oil, helianthus annuus (sunflower) seed oil, glycerin, glycyrrhiza glabra (licorice) root extract, tocopheryl acetate (vitamin E), panthenol (pro-vitamin B5), allantoin, zinc PCA, piroctone olamine, PEG-150 distearate, sodium citrate, acrylates crosspolymer-4, PEG-12, methyl glucose trioleate, phenoxyethanol, ethylhexylglycerin. *Certified Organic Ingredient - Mayacert Certifier
- QUESTIONS
-
SPL UNCLASSIFIED SECTION
Relieves & helps prevent recurrence of itching, flaking & scaling
• AP4 ® BOTANICAL • ALOE VERA COMPLEX
Psoriasis & Seborrheic Dermatitis
EXPERTS IN SKIN HEALING SINCE 1992
Psoriasis Control ® Shampoo contains clinically proven ingredients that help soothe and relieve psoriasis symptoms.
• Gentle on hair
• Relieves dry, itchy scalp & flaking
• Leaves hair silky softNo Steroids, Coal Tar or Cortisone Paraben & SLS Free
• Soothes dry, itchy scalp
• Relieves redness & flaking
QUALITY YOU CAN FEEL
RESULTS YOU CAN SEE. ®
TriDerma MD ® provides healing solutions for irritated skin without harmful side effects.
www.triderma.com
Dist. by: Genuine Virgin Aloe Corp.,
Corona, CA 92879 © 2019
MADE IN THE USA - Packaging
-
INGREDIENTS AND APPEARANCE
TRIDERMA PSORIASIS CONTROL
salicylic acid shampooProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10738-980 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 3 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SODIUM C14-16 OLEFIN SULFONATE (UNII: O9W3D3YF5U) COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) (1,1-DIMETHYLETHYL)UREA (UNII: T81TP489AF) GLYCOL DISTEARATE (UNII: 13W7MDN21W) POLYQUATERNIUM-7 (70/30 ACRYLAMIDE/DADMAC; 1600000 MW) (UNII: 0L414VCS5Y) OATMEAL (UNII: 8PI54V663Y) ALOE VERA LEAF (UNII: ZY81Z83H0X) EVENING PRIMROSE OIL (UNII: 3Q9L08K71N) SUNFLOWER OIL (UNII: 3W1JG795YI) GLYCERIN (UNII: PDC6A3C0OX) GLYCYRRHIZA GLABRA (UNII: 2788Z9758H) .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) PANTHENOL (UNII: WV9CM0O67Z) ALLANTOIN (UNII: 344S277G0Z) ZINC PIDOLATE (UNII: C32PQ86DH4) PIROCTONE OLAMINE (UNII: A4V5C6R9FB) PEG-150 DISTEARATE (UNII: 6F36Q0I0AC) SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) BUTYL ACRYLATE/METHYL METHACRYLATE/METHACRYLIC ACID COPOLYMER (18000 MW) (UNII: JZ1374NL9E) POLYETHYLENE GLYCOL 600 (UNII: NL4J9F21N9) METHYL GLUCOSE DIOLEATE (UNII: FA9KFJ4Z6P) PHENOXYETHANOL (UNII: HIE492ZZ3T) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10738-980-50 1 in 1 CARTON 01/15/2020 1 NDC:10738-980-55 15 mL in 1 TUBE; Type 0: Not a Combination Product 2 NDC:10738-980-82 245 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 01/15/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part358H 01/15/2020 Labeler - Genuine Virgin Aloe Corporation (961374147) Establishment Name Address ID/FEI Business Operations Genuine Virgin Aloe Corporation 961374147 manufacture(10738-980)