WAL ZYR- cetirizine hydrochloride tablet, film coated
Walgreen Company
----------
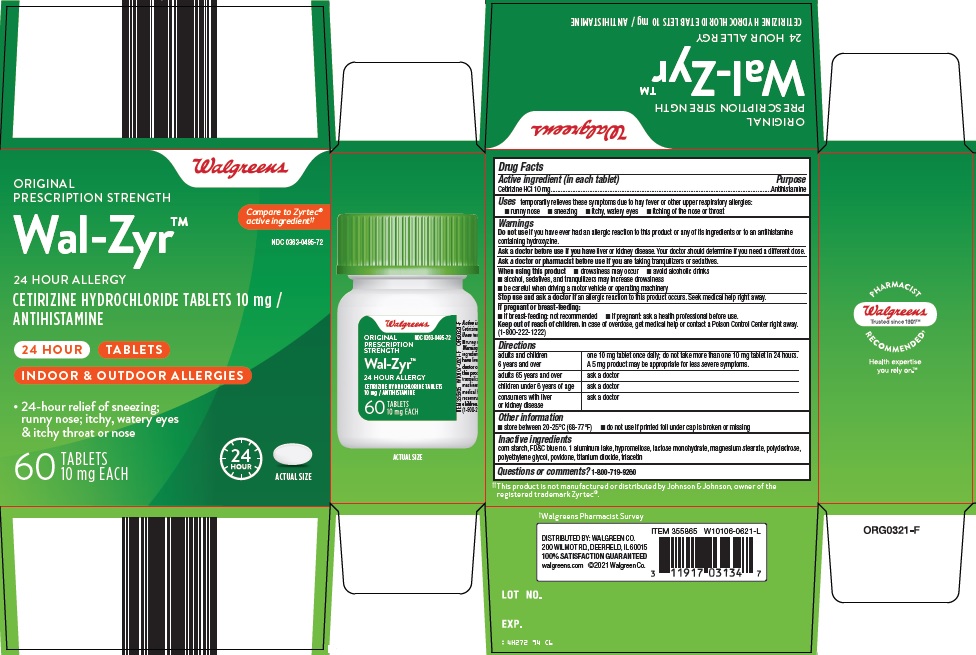
Walgreen Co. Wal-Zyr™ Drug Facts
Uses
temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- •
- runny nose
- •
- sneezing
- •
- itchy, watery eyes
- •
- itching of the nose or throat
Warnings
Do not use
if you have ever had an allergic reaction to this product or any of its ingredients or to an antihistamine containing hydroxyzine.
Ask a doctor before use if you have
liver or kidney disease. Your doctor should determine if you need a different dose.
When using this product
- •
- drowsiness may occur
- •
- avoid alcoholic drinks
- •
- alcohol, sedatives, and tranquilizers may increase drowsiness
- •
- be careful when driving a motor vehicle or operating machinery
Stop use and ask a doctor if
an allergic reaction to this product occurs. Seek medical help right away.
Directions
|
adults and children 6 years and over |
one 10 mg tablet once daily; do not take more than one 10 mg tablet in 24 hours. A 5 mg product may be appropriate for less severe symptoms. |
|
adults 65 years and over |
ask a doctor |
|
children under 6 years of age |
ask a doctor |
|
consumers with liver or kidney disease |
ask a doctor |
Other information
- •
- store between 20-25°C (68-77°F)
- •
- do not use if printed foil under cap is broken or missing
Inactive ingredients
corn starch, FD&C blue no. 1 aluminum lake, hypromellose, lactose monohydrate, magnesium stearate, polydextrose, polyethylene glycol, povidone, titanium dioxide, triacetin
Principal Display Panel
Walgreens
ORIGINAL PRESCRIPTION STRENGTH
Compare to Zyrtec® active ingredient
Wal-Zyr™
24 HOUR ALLERGY
CETIRIZINE HYDROCHLORIDE TABLETS 10 mg / ANTIHISTAMINE
24 HOUR
TABLETS
INDOOR & OUTDOOR ALLERGIES
• 24-hour relief of sneezing; runny nose; itchy, watery eyes & itchy throat or nose
60 TABLETS 10 mg EACH
24 HOUR
ACTUAL SIZE
| WAL ZYR
cetirizine hydrochloride tablet, film coated |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Walgreen Company (008965063) |