THERAPEUTIC- coal tar shampoo
CVS Pharmacy, Inc
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
CVS_497.004/497AH Coal tar dandruff shampoo
When using this product
- do not get into eyes. If contact occurs, rinse eyes thoroughly with water
- use caution in exposing skin to sunlight after applying this product. It may increase your tendency to sunburn for up to 24 hours after application.
- do not sue for prolonged periods without consulting a doctor
- do not use with other forms of psoriasis therapy such as ultraviolet radiation or prescription drugs unless directed to do so by a doctor.
Stop use and ask a doctor if
condition worsens or does not improve after reqular use of this product as directed.
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- for best results use at least twice a week or as directed by a doctor
- wet hair thoroughly
- massage shampoo into the scalp
- lather. Leave on hair and scalp for several minutes
- rinse and repeat
Other information
- store away from direct sunlight
- in rare instances, discoloration of gray, blonde, bleached or tinted hair may occur
Inactive ingredients
water, sodium laureth sulfate, cocamide MEA, cocamidopropyl betaine, polysorbate 20, laureth-4, bis-hydroxyethyl dihydroxypropyl stearammonium chloride, PEG-120 methyl glucose dioleate, tetrasodium EDTA, citric acid, methylchloroisothiazolinone, methylisothiazolinone, fragrance, caramel, red 4
CVS/pharmacy Extra Strength Therapeutic Dandruff Shampoo controls redness, itching and flaking caused by severe scalp conditions. Our active ingredient continues to provide effective scalp therapy even after you rinse.
Leaves hair shiny and manageable
Safe for regular use as directed
*This product is not manufactured or distributed by the Neutorgena Corporation, distributor of the registered trademark Neutrogena T/Gel.
Distributed by: CVS Pharmacy, Inc.
One CVS Drive, Woonsocket, RI 02895
2016 CVS/pharmacy
CVS.com 1-800-SHOP-CVS
Made in the U.S.A. with U. S. and foreign components
principal display panel
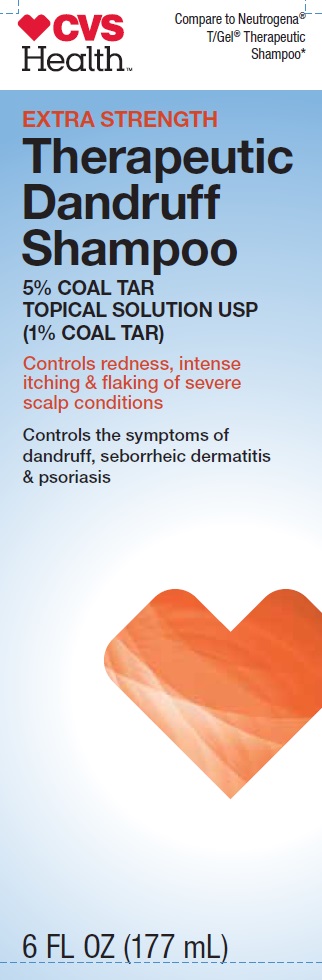
CVS Health
Compare to Neutrogena T/Gel Therapeutic Shampoo*
EXTRA STRENGTH
Therapeutic Dandruff Shampoo
5% COAL TAR TOPICAL SOLUTION USP (1% COAL TAR)
Controls redness, intense itching & flaking of severe scap conditions
Controls the symptoms of dandruff, seborrheic dermatitis & psoriasis
6 FL OZ (177 mL)
| THERAPEUTIC
coal tar shampoo |
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
| Labeler - CVS Pharmacy, Inc (062312574) |
| Registrant - Vi-Jon, LLC (790752542) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Vi-Jon, LLC | 790752542 | manufacture(59779-497) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Vi-Jon, LLC | 088520668 | manufacture(59779-497) | |