Label: EPSOM SALT granule
- NDC Code(s): 70677-0038-1, 70677-0038-2
- Packager: Strategic Sourcing Services LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated March 3, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Uses
-
Warnings
Ask a doctor before use if you have
- kidney disease
- a magnesium restricted diet
- stomach pain, nausea or vomiting
- noticed a sudden change in bowel habits that lasts more than 2 weeks
-
Directions
- do not exceed more than 2 doses per day; if necessary repeat dosage in 4 hours
age dose adults and children 12 years and older 2-4 level teaspoons dissolved in a full glass (8oz) of water children 6 to 11 years 1-2 level teaspoons dissolved in a full glass (8oz) of water
Not recommended for children under 6 years of age. - Other information
- Inactive ingredient
- Questions or comments?
-
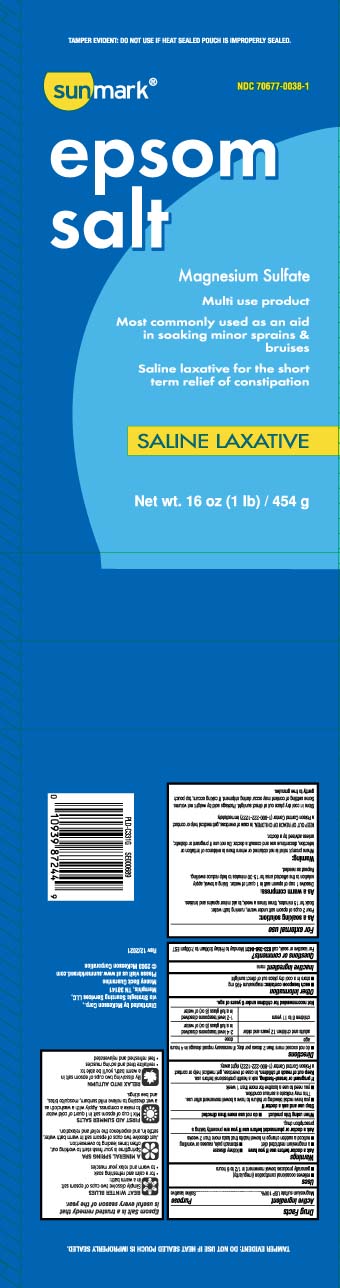
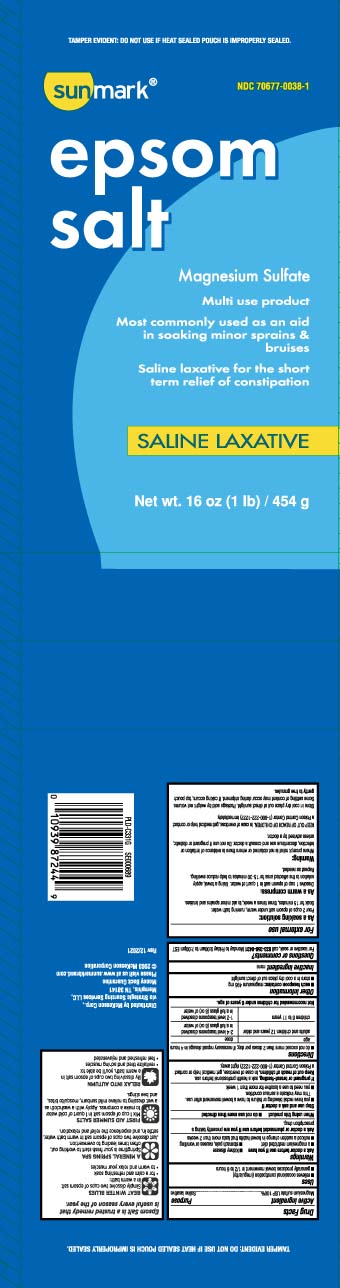
Principal Display Panel
Epsom salt
Magnesium Sulfate
Multi use product
Most commonly used as an aid
in soaking minor sprains & bruises
Saline laxative for the short term relief of constipation
SALINE LAXATIVE
Net Wt LB (kg)
*TAMPER EVIDENT: DO NOT USE IF HEAT SEALED POUCH IS IMPROPERLY SEALED.
Distributed by McKesson Corp.,
via Strategic Sourcing Services LLC,
Memphis, TN 38141
- Package Label
-
INGREDIENTS AND APPEARANCE
EPSOM SALT
epsom salt granuleProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:70677-0038 Route of Administration ORAL, TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MAGNESIUM SULFATE, UNSPECIFIED (UNII: DE08037SAB) (MAGNESIUM CATION - UNII:T6V3LHY838) MAGNESIUM SULFATE, UNSPECIFIED 1 g in 1 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70677-0038-2 1814 g in 1 POUCH; Type 0: Not a Combination Product 12/31/2017 2 NDC:70677-0038-1 454 g in 1 POUCH; Type 0: Not a Combination Product 12/31/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part334 12/31/2017 Labeler - Strategic Sourcing Services LLC (116956644)