ALCOHOL AND DEXTROSE- alcohol and dextrose monohydrate injection
B. Braun Medical Inc.
----------
Alcohol and Dextrose Injections USP
Hypertonic
DESCRIPTION
Each 100 mL of 5% Alcohol and 5% Dextrose Injection USP contains:
Alcohol USP 5 mL; Hydrous Dextrose USP 5 g
Water for Injection USP qs
pH: 5.0 (3.5–6.5); Calories per liter: 450
Calculated Osmolarity: 1125 mOsmol/liter, hypertonic
Each 100 mL of 10% Alcohol and 5% Dextrose Injection USP contains: Alcohol USP 10 mL; Hydrous Dextrose USP 5 g
Water for Injection USP qs
pH: 4.6 (3.5–6.5); Calories per liter: 720
Calculated Osmolarity: 1995 mOsmol/liter, strongly hypertonic
These intravenous solutions are sterile, nonpyrogenic, hypertonic and contain no bacteriostatic or antimicrobial agents.
The formulas of the active ingredients are:
| Ingredients | Molecular Formula | MolecularWeight |
| Alcohol USP | CH3CH2OH | 46.07 |
| Hydrous Dextrose USP | 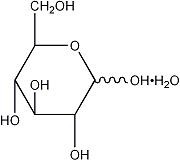 | 198.17 |
CLINICAL PHARMACOLOGY
Alcohol and Dextrose Injections USP are an intravenous source of calories. In the average adult, pure ethyl alcohol is metabolized at a rate of 10 to 20 mL per hour. Sedative effects of alcohol occur if the rate of infusion exceeds the rate of metabolism. Dextrose (D-glucose) can be infused at a maximum rate of approximately 0.5 to 0.85 g/kg of body weight/hr without producing significant glycosuria. Thus, the maximum rate that alcohol can be infused without producing sedative effects is well below the maximum rate of utilization of dextrose.
Alcohol is metabolized, mostly in the liver, to acetaldehyde or acetate. The rate of oxidation is a linear function of time. Starvation lowers the rate of metabolism and insulin increases the rate.
INDICATIONS AND USAGE
Alcohol and Dextrose Injections USP are indicated for increasing caloric intake.
CONTRAINDICATIONS
Alcohol should not be used in patients with epilepsy, urinary tract infection, or diabetic coma.
Alcohol is contraindicated in patients who have been addicted to it.
Do not give subcutaneously and avoid extravasation during intravenous administration.
Solutions containing dextrose may be contraindicated in patients with hypersensitivity to corn products.
WARNINGS
Alcohol should be used cautiously, if at all, in patients with liver impairment, in the presence of shock, following cranial surgery, in actual or anticipated postpartum hemorrhage, or in the presence of significant renal impairment.
Alcohol decreases blood sugar in diabetic patients. In the untreated diabetic, the rate of alcohol metabolism is slowed.
As a nutrient, alcohol supplies only calories. Given alone, it may cause or potentiate vitamin deficiencies and certain liver alterations.
Alcohol crosses the placenta rapidly and enters the fetal circulation. It may also be found in the milk of lactating women. The use of these solutions in pregnancy should be carefully considered.
PRECAUTIONS
General
Alcohol and Dextrose Injections USP should be administered slowly, and the patient observed for restlessness or narcosis.
The half lives of phenytoin, warfarin and tolbutamide may be shortened 50% to 75% by concurrent administration of alcohol. Alcohol increases serum uric acid and can precipitate acute gout.
The vasodilating effect of alcohol may potentiate postural hypotension, particularly in association with some antihypertensive drugs.
If the administration is controlled by a pumping device, care must be taken to discontinue pumping action before the container runs dry or air embolism may result.
To minimize the risk of possible incompatibilities arising from mixing this solution with other additives that may be prescribed, the final infusate should be inspected for cloudiness or precipitation immediately after mixing, prior to administration, and periodically during administration.
Use only if solution is clear and vacuum is present.
Usage in Pregnancy
Pregnancy Category C
Animal reproduction studies have not been conducted with Alcohol and Dextrose Injections USP. It is also not known whether Alcohol and Dextrose Injections USP can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Alcohol and Dextrose Injections USP should be given to a pregnant woman only if clearly needed.
ADVERSE REACTIONS
Alcoholic intoxication may occur with too rapid infusion. Vertigo, flushing, disorientation (especially in elderly patients), or sedation may also occur. An alcoholic odor may be noted in the breath. Generally, these effects can be avoided by slowing the rate of infusion.
Too rapid infusion of hypertonic solutions may cause local pain and, rarely, vein irritation. Use of the largest available peripheral vein and a well-placed, small bore needle is recommended.
OVERDOSAGE
In the event of fluid overload during parenteral therapy, reevaluate the patient's condition, and institute appropriate corrective treatment.
DOSAGE AND ADMINISTRATION
Alcohol and Dextrose Injections USP are administered by intravenous infusion only. Total dosage and rate of infusion depend on the patient's response and tolerance. The average adult can metabolize approximately 10 mL of pure alcohol per hour, equivalent to the alcohol contained in 200 mL of a 5% solution or 100 mL of a 10% solution. The usual adult dosage is 1 to 2 liters and rarely exceeds 3 liters of a 5% solution in a 24-hour period. Children may be given 40 mL per kg per 24 hours or from 350 mL to 1000 mL depending on size and clinical response.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit.
HOW SUPPLIED
These solutions are supplied sterile and nonpyrogenic in single dose glass containers packaged 6 per case.
| CanadaDIN | NDC | Cat. No. | Size |
| 10% Alcohol and 5% Dextrose Injection USP | |||
| 0264-1978-00 | S9780 | 1000 mL | |
| 5% Alcohol and 5% Dextrose Injection USP | |||
| 01924230 | 0264-1981-00 | S9810 | 1000 mL |
Exposure of pharmaceutical products to heat should be minimized. Avoid excessive heat. Protect from freezing. It is recommended that the product be stored at room temperature (25°C); however, brief exposure up to 40°C does not adversely affect the product.
Directions for Use of B. Braun Glass Containers
Before use, perform the following checks:
- Inspect each container. Read the label. Ensure solution is the one ordered and is within the expiration date. Check the security of bail and band.
- Invert container and carefully inspect the solution in good light for cloudiness, haze, or particulate matter; check the bottle for cracks or other damage. In checking for cracks, do not be confused by normal surface marks and seams on bottom and sides of bottle. These are not flaws. Look for bright reflections that have depth and penetrate into the wall of the bottle. Reject any such bottle.
-
To remove the outer closure, lift the tear tab and pull up, over, and down until it is below the stopper (See Figure 1). Use a circular pulling motion on the tab until it breaks away.
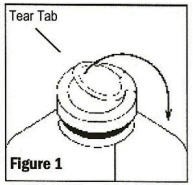
-
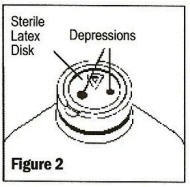
Grasp and remove the metal disk, exercising caution not to touch the sterile latex disk underneath.
-
With the sterile latex disk exposed, check for vacuum by confirming the presence of depressions in the latex disk, which should be held tightly over stopper (See Figure 2). If the latex disk is puffed or depressions cannot be seen, the vacuum has dissipated and the bottle should be rejected. The sterile latex disk provides a surface for aseptic medication addition prior to administration.
Note: When vacuum is essential for the use of the product (medication addition or transfer, etc.) the latex disk should be left in place until all additions or transfers are completed. Medication addition or transfers should be made immediately after exposing the sterile latex disk. Identify three depressions in the latex disk prior to adding medication: a triangular medication site, one large round outlet port, and one small air-inletting port (See Figures 2 and 3).
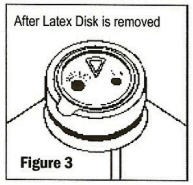
-
Before removing the latex disk, add medication through the triangular (
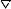 ) medication site (See Figure 4). The vacuum in the container will automatically draw the contents of a syringe or spiked vial into the container. Each addition/transfer will reduce the vacuum remaining in the bottle.
) medication site (See Figure 4). The vacuum in the container will automatically draw the contents of a syringe or spiked vial into the container. Each addition/transfer will reduce the vacuum remaining in the bottle.
Warning: Some additives may be incompatible. Consult with pharmacist. When introducing additives, use aseptic techniques. Mix thoroughly.
Do not store. -
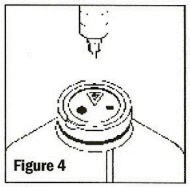
Remove the latex disk prior to inserting administration set. To remove the latex disk, grasp the lip of the disk, lift and pull up and away (See Figure 5). As the disk is lifted, and if no additions have been made, vacuum can be confirmed by an audible hiss.
-
Refer to Directions for Use of the set being used. Insert the set spike into the large round outlet port of the stopper and hang container.
-
After admixture and during administration, reinspect the solution frequently. If any evidence of solution contamination or instability is found or if the patient exhibits any signs of fever, chills or other reactions not readily explainable, discontinue administration immediately and notify the physician.
-
When adding medication to the container during administration, swab the triangular medication site, inject medication and mix thoroughly by gentle agitation.
B. Braun Medical Inc.
Irvine, CA USA 92614-5895
In Canada, distributed by:
B. Braun Medical Inc.
Scarborough, Ontario M1H 2W4
©1999 B. Braun Medical Inc.
Y36-002-423
PRINCIPAL DISPLAY PANEL - CONTAINER LABEL
1000 mL
NDC 0264-1978-00
S9780
10% Alcohol and 5% Dextrose Injection USP
Hypertonic
Each 100 mL contains:
Alcohol USP 10 mL
Hydrous Dextrose USP 5 g
Water for Injection USP qs
pH: 4.6 (3.5-6.5)
Calc. Osmolarity: 1995 mOsmol/liter
Strongly Hypertonic
Sterile, nonpyrogenic. Single dose container.
For intravenous use only. Use only if solution
is clear and vacuum is present.
Warning: Inspect venipuncture frequently to
detect and avoid infiltration.
Recommended Storage:
Room temperature (25°C). Avoid excessive
heat. Protect from freezing. See Package Insert.
Rx only
B. Braun Medical Inc.
Irvine, CA USA 92614-5895
Y37-001-868
Lot
Exp.

PRINCIPAL DISPLAY PANEL - CONTAINER LABEL
1000 mL
Canada DIN 01924230
NDC 0264-1981-00
S9810
5% Alcohol and 5% Dextrose Injection USP
Hypertonic
Each 100 mL contains:
Alcohol USP 5 mL
Hydrous Dextrose USP 5 g
Water for Injection USP qs
pH: 5.0 (3.5-6.5)
Calc. Osmolarity: 1125 mOsmol/liter, Hypertonic
Sterile, nonpyrogenic. Single dose container.
For intravenous use only. Use only if solution
is clear and vacuum is present.
Warning: Inspect venipuncture frequently to
detect and avoid infiltration.
Recommended Storage:
Room temperature (25°C). Avoid excessive
heat. Protect from freezing. See Package Insert.
Rx only
B. Braun Medical Inc.
Irvine, CA USA 92614-5895
In Canada, distributed by:
B. Braun Medical Inc.
Scarborough, Ontario M1H 2W4
Y37-001-869
Lot
Exp.

| ALCOHOL AND DEXTROSE
alcohol and dextrose monohydrate injection |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| ALCOHOL AND DEXTROSE
alcohol and dextrose monohydrate injection |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - B. Braun Medical Inc. (002397347) |