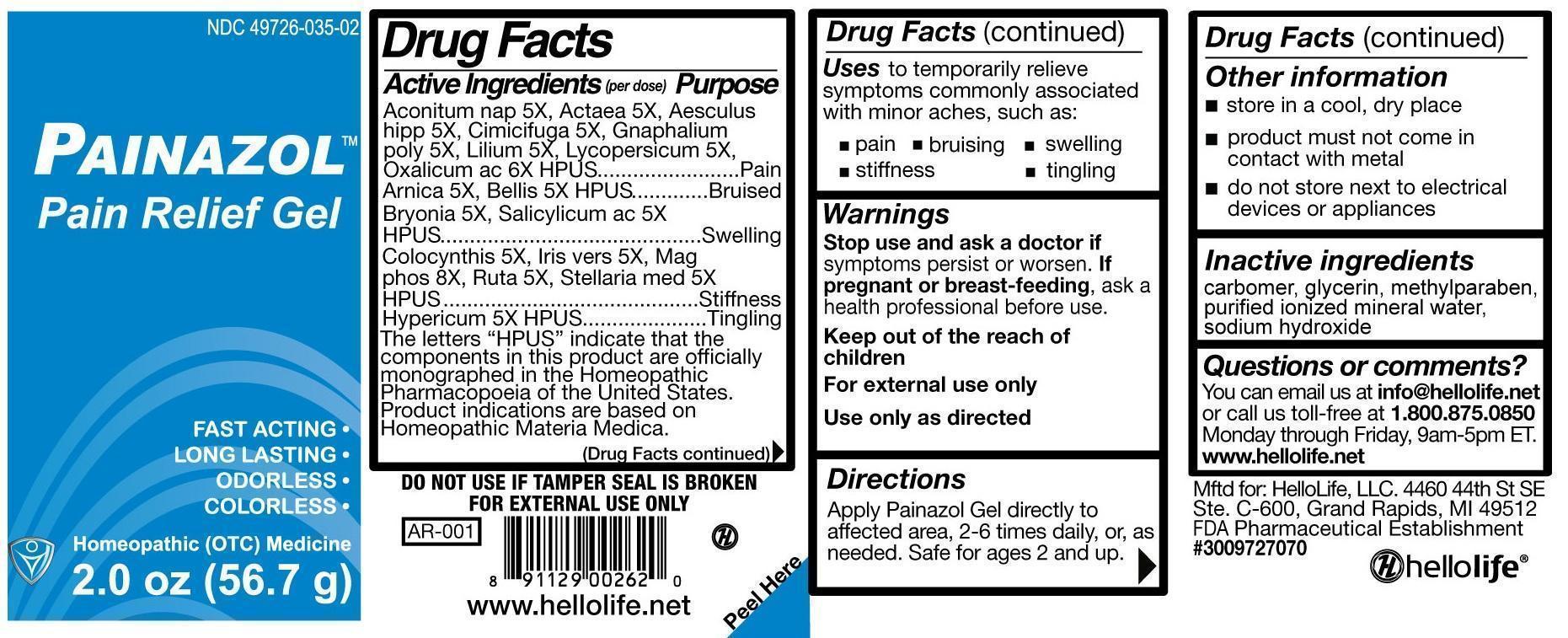
PAINAZOL PAIN RELIEF- aconitum nap, actaea, aesculus hipp, cimicifuga, gnaphalium poly, lilium, lycopersicum, oxalicum ac, arnica, bellis, bryonia, salicylicum ac, colocynthis, iris vers, mag phos, ruta, stellaria med, hypericum gel
Hello Life, Inc.
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
Drug Facts
Active Ingredients (per dose)
Aconitum nap 5X, Actaea 5X, Aesculus
hipp 5X, Cimicifuga 5X, Gnaphalium
poly 5X, Lilium 5X, Lycopersicum 5X,
Oxalicum ac 6X HPUS
Arnica 5X, Bellis 5X HPUS
Bryonia 5X, Salicylicum ac 5X HPUS
Colocynthis 5X, Iris vers 5X, Mag
phos 8X, Ruta 5X, Stellaria med 5X HPUS
Hypericum 5X HPUS
Purpose
Aconitum nap 5X, Actaea 5X, Aesculus
hipp 5X, Cimicifuga 5X, Gnaphalium
poly 5X, Lilium 5X, Lycopersicum 5X,
Oxalicum ac 6X HPUS……………………………..…Pain
Arnica 5X, Bellis 5X HPUS……….……………….....Bruised
Bryonia 5X, Salicylicum ac 5X HPUS………….......Swelling
Colocynthis 5X, Iris vers 5X, Mag
phos 8X, Ruta 5X, Stellaria med 5X HPUS….........Stiffness
Hypericum 5X HPUS……………………………….....Tingling
The letters “HPUS” indicate that the components in this product are officially monographed in the Homeopathic Pharmacopoeia of the United States. Product indications are based on Homeopathic Materia Medica.
Uses
to temporarily relieve symptoms commonly associated with minor aches, such as:
• pain
• bruising
• swelling
• stiffness
• tingling
Directions
Apply Painazol Gel directly to affected area, 2-6 times daily, or, as needed. Safe for ages 2 and up.
Other information
• store in a cool, dry place
• product must not come in contact with metal
• do not store next to electrical devices or appliances
Inactive ingredients
carbomer, glycerin, methylparaben, purified ionized mineral water, sodium hydroxide
Questions or comments?
You can email us at info@hellolife.net or call us toll-free at
1.800.875.0850. Monday through Friday, 9am-5pm ET.
www.hellolife.net
| PAINAZOL PAIN RELIEF
aconitum nap, actaea, aesculus hipp, cimicifuga, gnaphalium poly, lilium, lycopersicum, oxalicum ac, arnica, bellis, bryonia, salicylicum ac, colocynthis, iris vers, mag phos, ruta, stellaria med, hypericum gel |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Hello Life, Inc. (065619378) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Cosmetic Specialty Labs, Inc. | 032973000 | manufacture(49726-035) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Hello Life, Inc. | 079860489 | relabel(49726-035) , repack(49726-035) | |