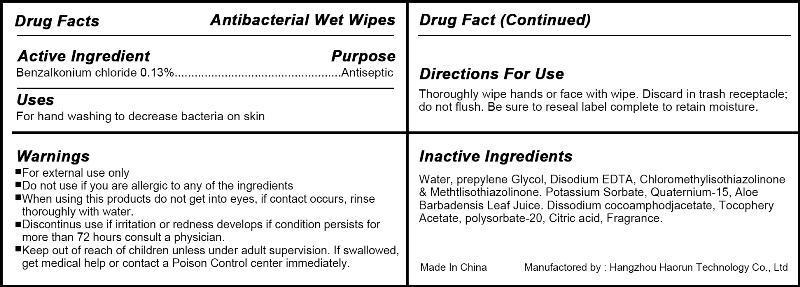
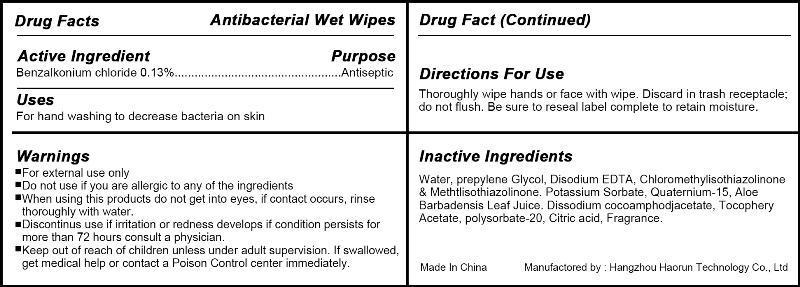
Label: ANTIBACTERIAL WET WIPES- benzalkonium chloride swab
-
Contains inactivated NDC Code(s)
NDC Code(s): 57817-100-01, 57817-100-02, 57817-100-03, 57817-100-04, view more57817-100-05, 57817-100-06, 57817-100-07, 57817-100-08, 57817-100-09, 57817-100-10, 57817-100-11, 57817-100-12, 57817-100-13, 57817-100-14, 57817-100-15, 57817-100-16, 57817-100-17, 57817-100-18, 57817-100-19, 57817-100-20, 57817-100-21, 57817-100-22, 57817-100-23, 57817-100-24, 57817-100-25, 57817-100-26, 57817-100-27, 57817-100-28, 57817-100-29, 57817-100-30, 57817-100-31, 57817-100-32, 57817-100-33, 57817-100-34, 57817-100-35, 57817-100-36, 57817-100-37, 57817-100-38, 57817-100-39, 57817-100-40, 57817-100-41, 57817-100-42, 57817-100-43, 57817-100-44, 57817-100-45, 57817-100-46, 57817-100-47, 57817-100-48, 57817-100-49, 57817-100-50, 57817-100-51, 57817-100-52, 57817-100-53, 57817-100-54, 57817-100-55, 57817-100-56, 57817-100-57, 57817-100-58, 57817-100-59, 57817-100-60, 57817-100-61, 57817-100-62, 57817-100-63, 57817-100-64, 57817-100-65, 57817-100-66 - Packager: Hangzhou Haorun Technology CO.,LTD.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated March 29, 2013
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Use
- WARNINGS
- Directions
- Inactive ingredients
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ANTIBACTERIAL WET WIPES
benzalkonium chloride swabProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:57817-100 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Benzalkonium Chloride (UNII: F5UM2KM3W7) (Benzalkonium - UNII:7N6JUD5X6Y) Benzalkonium Chloride 0.13 g in 100 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) OCTHILINONE (UNII: 4LFS24GD0V) .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:57817-100-01 1 in 1 BOTTLE 1 10 g in 1 POUCH 2 NDC:57817-100-02 2 in 1 BOTTLE 2 8 g in 1 POUCH 3 NDC:57817-100-03 3 in 1 BOTTLE 3 6 g in 1 POUCH 4 NDC:57817-100-04 4 in 1 BOTTLE 4 5 g in 1 POUCH 5 NDC:57817-100-05 5 in 1 BOTTLE 5 5 g in 1 POUCH 6 NDC:57817-100-06 6 in 1 BOTTLE 6 5 g in 1 POUCH 7 NDC:57817-100-07 7 in 1 BOTTLE 7 5 g in 1 POUCH 8 NDC:57817-100-08 8 in 1 BOTTLE 8 5 g in 1 POUCH 9 NDC:57817-100-09 9 in 1 BOTTLE 9 5 g in 1 POUCH 10 NDC:57817-100-10 10 in 1 BOTTLE 10 3.5 g in 1 POUCH 11 NDC:57817-100-11 12 in 1 BOTTLE 11 3.5 g in 1 POUCH 12 NDC:57817-100-12 15 in 1 BOTTLE 12 3 g in 1 POUCH 13 NDC:57817-100-13 18 in 1 BOTTLE 13 3 g in 1 POUCH 14 NDC:57817-100-14 20 in 1 BOTTLE 14 3 g in 1 POUCH 15 NDC:57817-100-15 25 in 1 BOTTLE 15 3 g in 1 POUCH 16 NDC:57817-100-16 30 in 1 BOTTLE 16 2.5 g in 1 POUCH 17 NDC:57817-100-17 35 in 1 BOTTLE 17 2.5 g in 1 POUCH 18 NDC:57817-100-18 40 in 1 BOTTLE 18 2.5 g in 1 POUCH 19 NDC:57817-100-19 45 in 1 BOTTLE 19 2.5 g in 1 POUCH 20 NDC:57817-100-20 50 in 1 BOTTLE 20 2.5 g in 1 POUCH 21 NDC:57817-100-21 60 in 1 BOTTLE 21 2.5 g in 1 POUCH 22 NDC:57817-100-22 72 in 1 BOTTLE 22 2.5 g in 1 POUCH 23 NDC:57817-100-23 80 in 1 BOTTLE 23 2.5 g in 1 POUCH 24 NDC:57817-100-24 90 in 1 BOTTLE 24 2.5 g in 1 POUCH 25 NDC:57817-100-25 100 in 1 BOTTLE 25 2.5 g in 1 POUCH 26 NDC:57817-100-26 110 in 1 BOTTLE 26 2.5 g in 1 POUCH 27 NDC:57817-100-27 120 in 1 BOTTLE 27 2.5 g in 1 POUCH 28 NDC:57817-100-28 150 in 1 BOTTLE 28 2.5 g in 1 POUCH 29 NDC:57817-100-29 180 in 1 BOTTLE 29 2.5 g in 1 POUCH 30 NDC:57817-100-30 200 in 1 BOTTLE 30 2.5 g in 1 POUCH 31 NDC:57817-100-31 250 in 1 BOTTLE 31 2.5 g in 1 POUCH 32 NDC:57817-100-32 300 in 1 BOTTLE 32 2.5 g in 1 POUCH 33 NDC:57817-100-33 400 in 1 BOTTLE 33 2.5 g in 1 POUCH 34 NDC:57817-100-34 1 in 1 PACKAGE 34 3 g in 1 POUCH 35 NDC:57817-100-35 2 in 1 PACKAGE 35 3 g in 1 POUCH 36 NDC:57817-100-36 3 in 1 PACKAGE 36 3 g in 1 POUCH 37 NDC:57817-100-37 4 in 1 PACKAGE 37 3 g in 1 POUCH 38 NDC:57817-100-38 5 in 1 PACKAGE 38 3 g in 1 POUCH 39 NDC:57817-100-39 6 in 1 PACKAGE 39 3.5 g in 1 POUCH 40 NDC:57817-100-40 7 in 1 PACKAGE 40 3.5 g in 1 POUCH 41 NDC:57817-100-41 8 in 1 PACKAGE 41 3.5 g in 1 POUCH 42 NDC:57817-100-42 9 in 1 PACKAGE 42 3.5 g in 1 POUCH 43 NDC:57817-100-43 10 in 1 PACKAGE 43 4 g in 1 POUCH 44 NDC:57817-100-44 12 in 1 PACKAGE 44 4 g in 1 POUCH 45 NDC:57817-100-45 15 in 1 PACKAGE 45 4 g in 1 POUCH 46 NDC:57817-100-46 18 in 1 PACKAGE 46 4 g in 1 POUCH 47 NDC:57817-100-47 20 in 1 PACKAGE 47 4 g in 1 POUCH 48 NDC:57817-100-48 25 in 1 PACKAGE 48 4 g in 1 POUCH 49 NDC:57817-100-49 30 in 1 PACKAGE 49 4 g in 1 POUCH 50 NDC:57817-100-50 35 in 1 PACKAGE 50 4 g in 1 POUCH 51 NDC:57817-100-51 40 in 1 PACKAGE 51 4 g in 1 POUCH 52 NDC:57817-100-52 45 in 1 PACKAGE 52 4 g in 1 POUCH 53 NDC:57817-100-53 50 in 1 PACKAGE 53 4 g in 1 POUCH 54 NDC:57817-100-54 60 in 1 PACKAGE 54 4 g in 1 POUCH 55 NDC:57817-100-55 72 in 1 PACKAGE 55 4 g in 1 POUCH 56 NDC:57817-100-56 80 in 1 PACKAGE 56 4 g in 1 POUCH 57 NDC:57817-100-57 90 in 1 PACKAGE 57 4 g in 1 POUCH 58 NDC:57817-100-58 100 in 1 PACKAGE 58 4 g in 1 POUCH 59 NDC:57817-100-59 110 in 1 PACKAGE 59 4 g in 1 POUCH 60 NDC:57817-100-60 120 in 1 PACKAGE 60 4 g in 1 POUCH 61 NDC:57817-100-61 150 in 1 PACKAGE 61 4 g in 1 POUCH 62 NDC:57817-100-62 180 in 1 PACKAGE 62 4 g in 1 POUCH 63 NDC:57817-100-63 200 in 1 PACKAGE 63 4 g in 1 POUCH 64 NDC:57817-100-64 250 in 1 PACKAGE 64 4 g in 1 POUCH 65 NDC:57817-100-65 350 in 1 PACKAGE 65 4 g in 1 POUCH 66 NDC:57817-100-66 400 in 1 PACKAGE 66 4 g in 1 POUCH Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 03/29/2013 Labeler - Hangzhou Haorun Technology CO.,LTD. (421308583) Establishment Name Address ID/FEI Business Operations Hangzhou Haorun Technology CO.,LTD. 421308583 manufacture(57817-100)