AFTERTEST PAIN RELIEF- benzocaine stick
Diabetic Supply of Suncoast, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Aftertest Pain Relief
Uses
Temporarily relieves pain and itching, associated with: minor cuts, scrapes, minor burns, insect bites.
Directions
When practical, cleanse affected area with mild soap and warm water and rinse thoroughly. Gently dry by patting or blotting with toilet tissue or soft cloth before application of this product. Apply externally to the affected area up to 6 times daily.
Inactive Ingredient
Alcohol Denat., Amica Montana Flower Extract, Diisopropyl Adipate, Eucalyptus Globulus Leaf Oil, Glycerin, Glycery Laurate, Menthol, Polyacrylate-13, Polyisobutene, Polysorbate 20, Potassium Laurate, Propanediol, Propylene Glycol, Propylene Glycol Dilaurate, Propylene Glycol Monolaurate, Water.
Store at room temperature 15-30 degrees C (59-86 degrees F). Do not freeze.
Throw away any unused medicine after the expiration date.
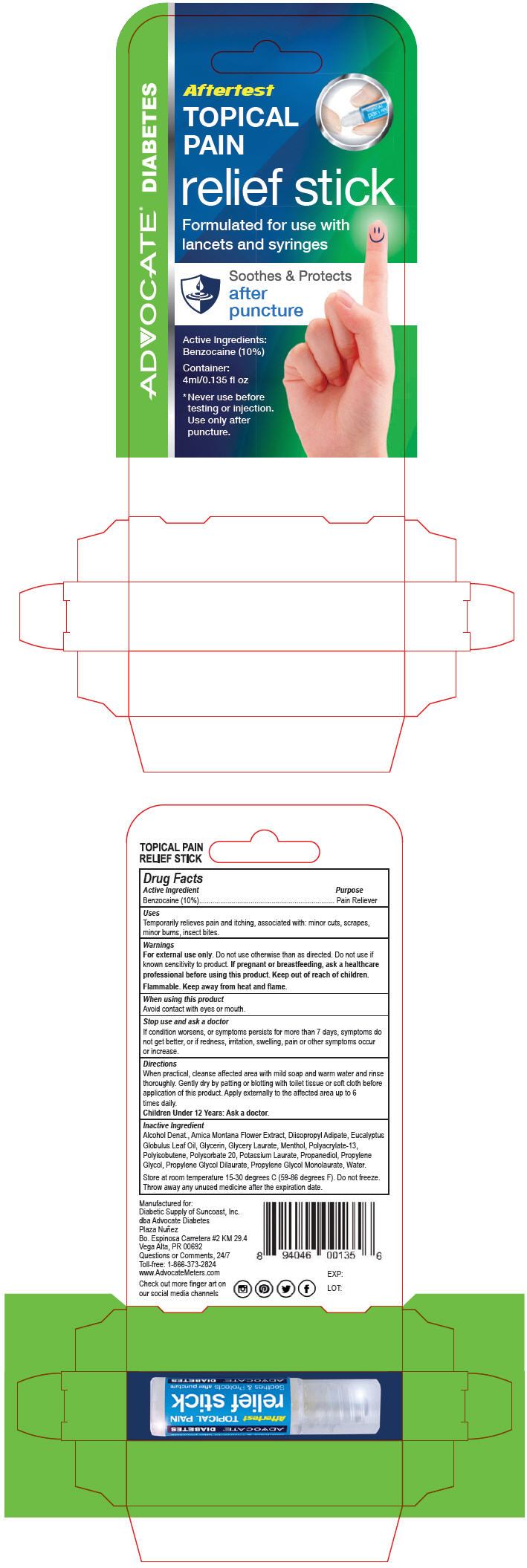
PRINCIPAL DISPLAY PANEL - 4 ml Applicator Carton
Aftertest
TOPICAL
PAIN
relief stick
Formulated for use with
lancets and syringes
Soothes & Protects
after
puncture
Active Ingredients:
Benzocaine (10%)
Container:
4ml/0.135 fl oz
* Never use before
testing or injection.
Use only after
puncture.
ADVOCATE ® DIABETES
| AFTERTEST PAIN RELIEF
benzocaine stick |
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
| Labeler - Diabetic Supply of Suncoast, Inc. (043081723) |