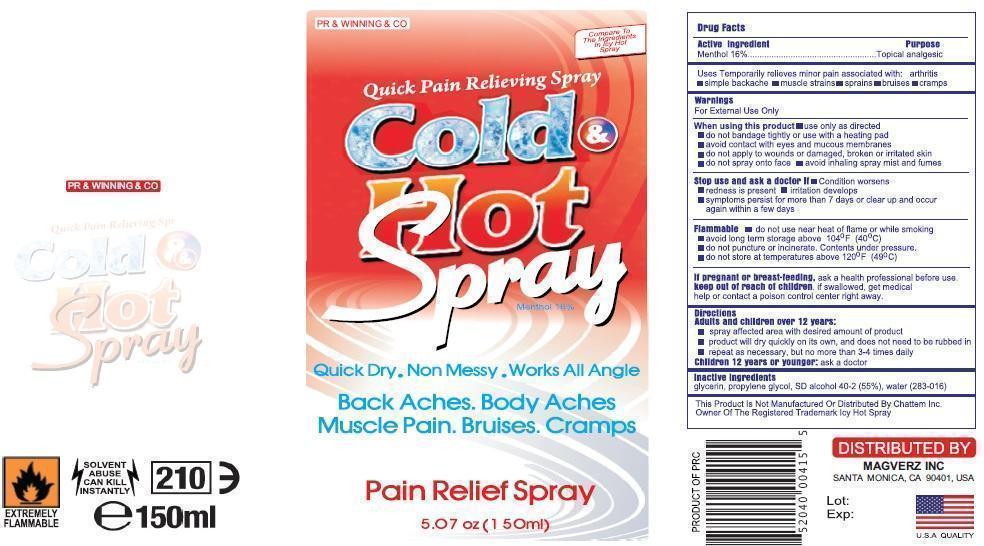
Label: COLD AND HOT- menthol spray
-
Contains inactivated NDC Code(s)
NDC Code(s): 59240-002-10 - Packager: MAGVERZ INC
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated July 13, 2013
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Acitves
- Inactives
- Uses
- Indication
- Directions
-
Warnings
For External use only
When using this product
Use only as directed
Do not bandage tightly or with heating pad
Avoid contact with eyes and mucous membranes
Do not apply to wounds or damagged, broken or irritated skin
Do not spray on face
Avoid inhaling spray mist and fumes
Stop use and ask docotor if;
Condition Worsesns
Redness is presnt for more than 7 days
Irritation developes
Symptomps persist for mor than 7 dyas or clear up and occur again within a few days.
Flamalble
Do not use near heat or flame or while smoking.Avod long term storage above 104oF (400C)
Do not puncture of or incinerate.Congents under pressure.
Do not store at temreture above 120oF (490C)
If pregnant or breast feeding
Ask health professionals before use.
If sollowed get medical help or contact poison control center right away.
- Keep Out of Reach of Childrens
- Productt Label
-
INGREDIENTS AND APPEARANCE
COLD AND HOT
menthol sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59240-002 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 16 g in 100 mL Inactive Ingredients Ingredient Name Strength METHYL SALICYLATE (UNII: LAV5U5022Y) THYMOL (UNII: 3J50XA376E) ZINC STEARATE (UNII: H92E6QA4FV) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59240-002-10 150 mL in 1 CAN Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 07/18/2013 Labeler - MAGVERZ INC (078712269) Registrant - MAGVERZ INC (078712269)