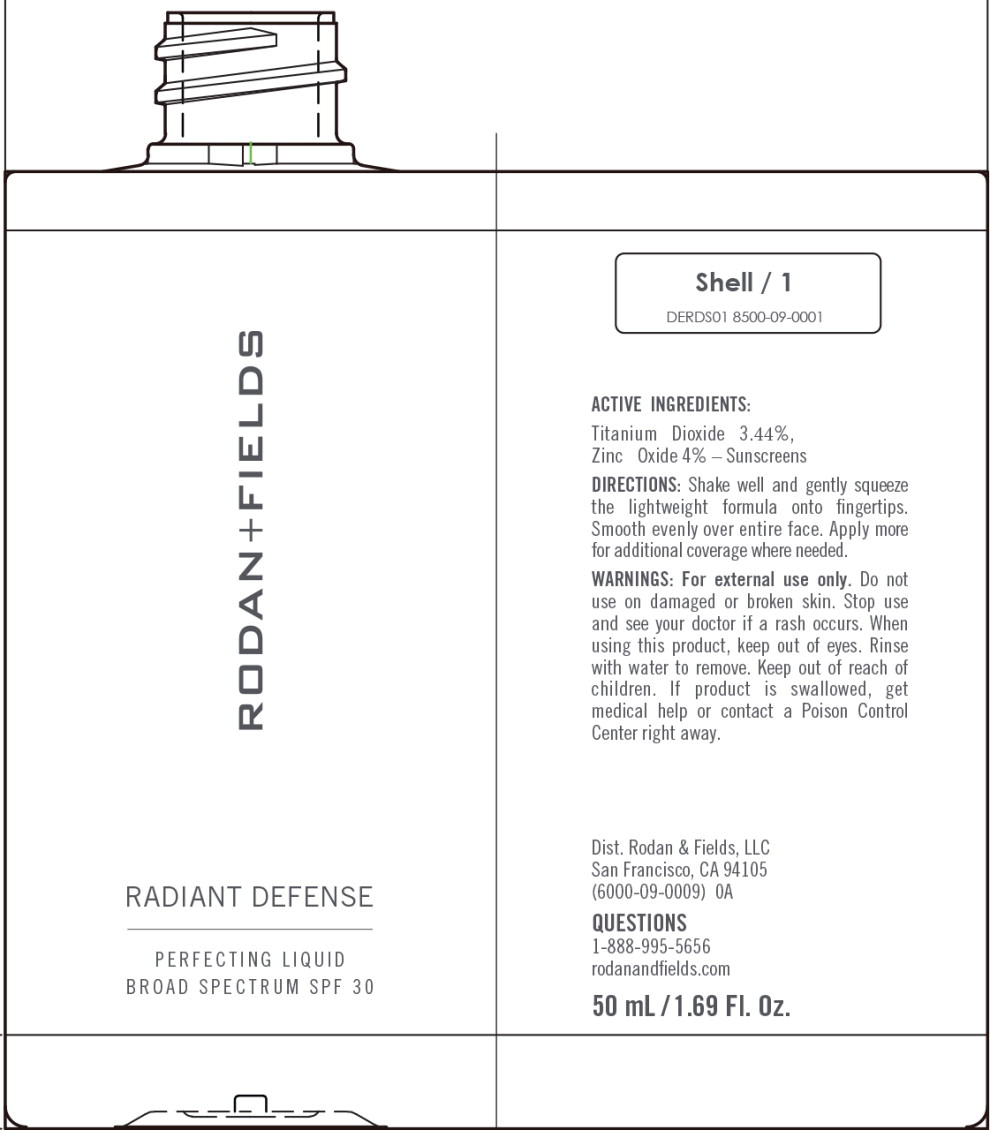
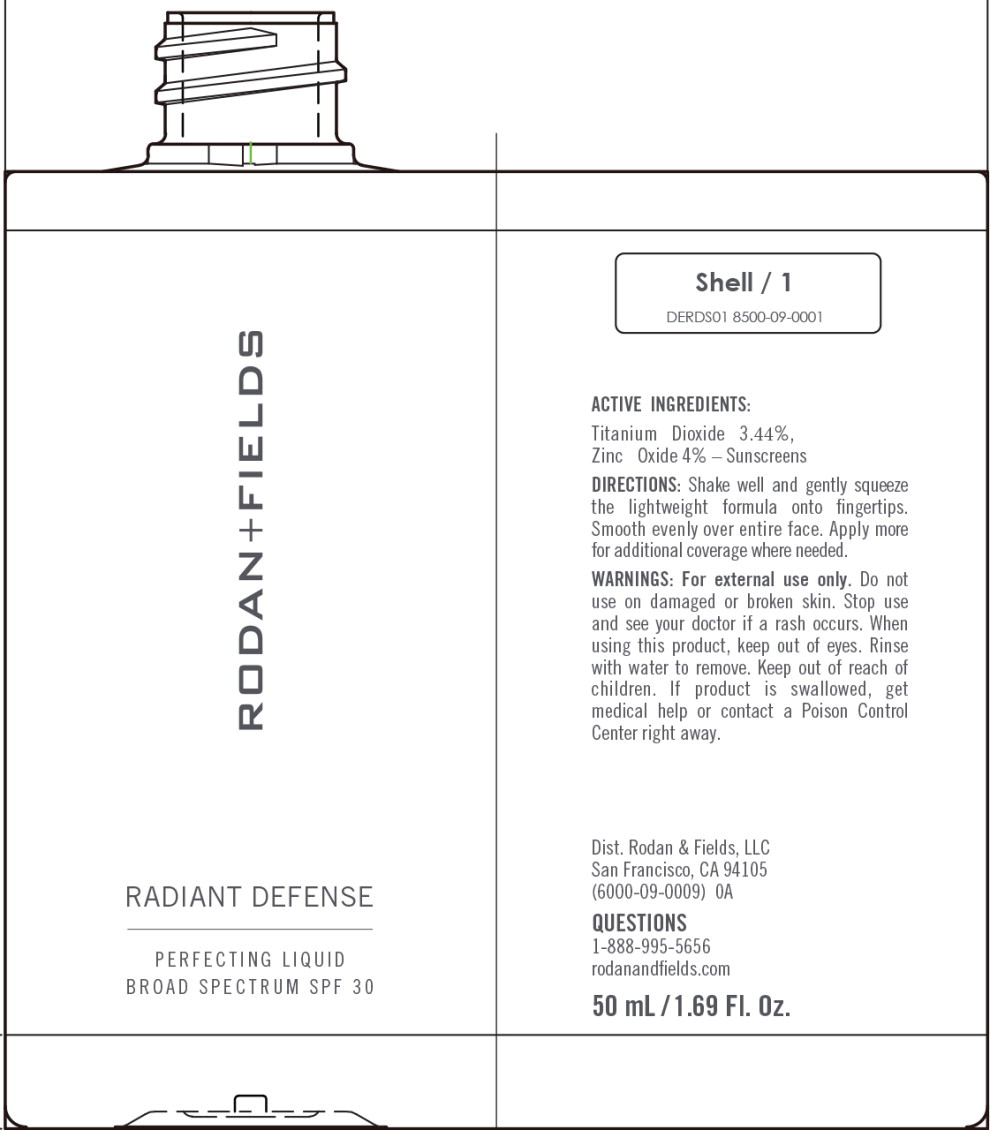
Label: RADIANT DEFENSE PERFECTING LIQUID BROAD SPECTRUM SPF 30 - SHELL- titanium dioxide, zinc oxide lotion
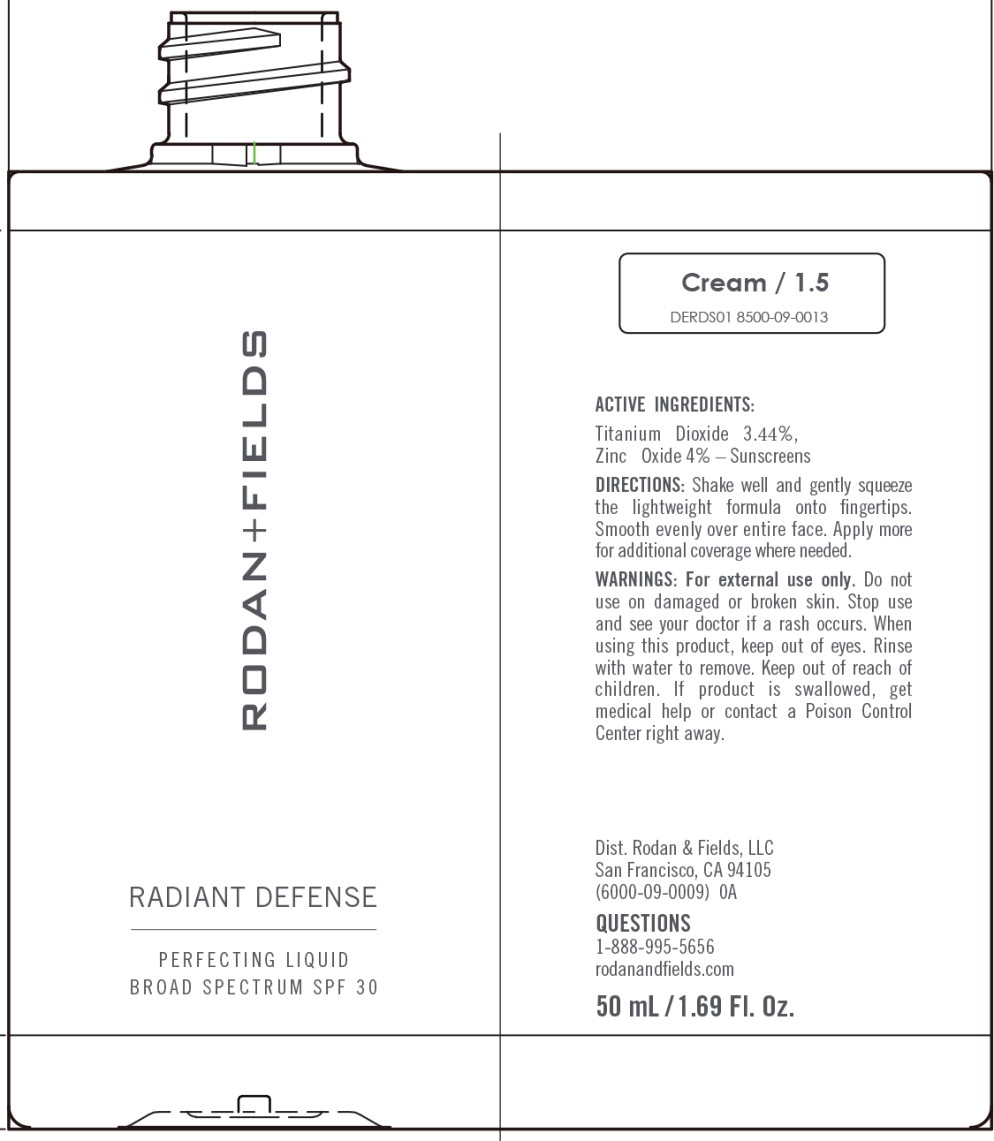
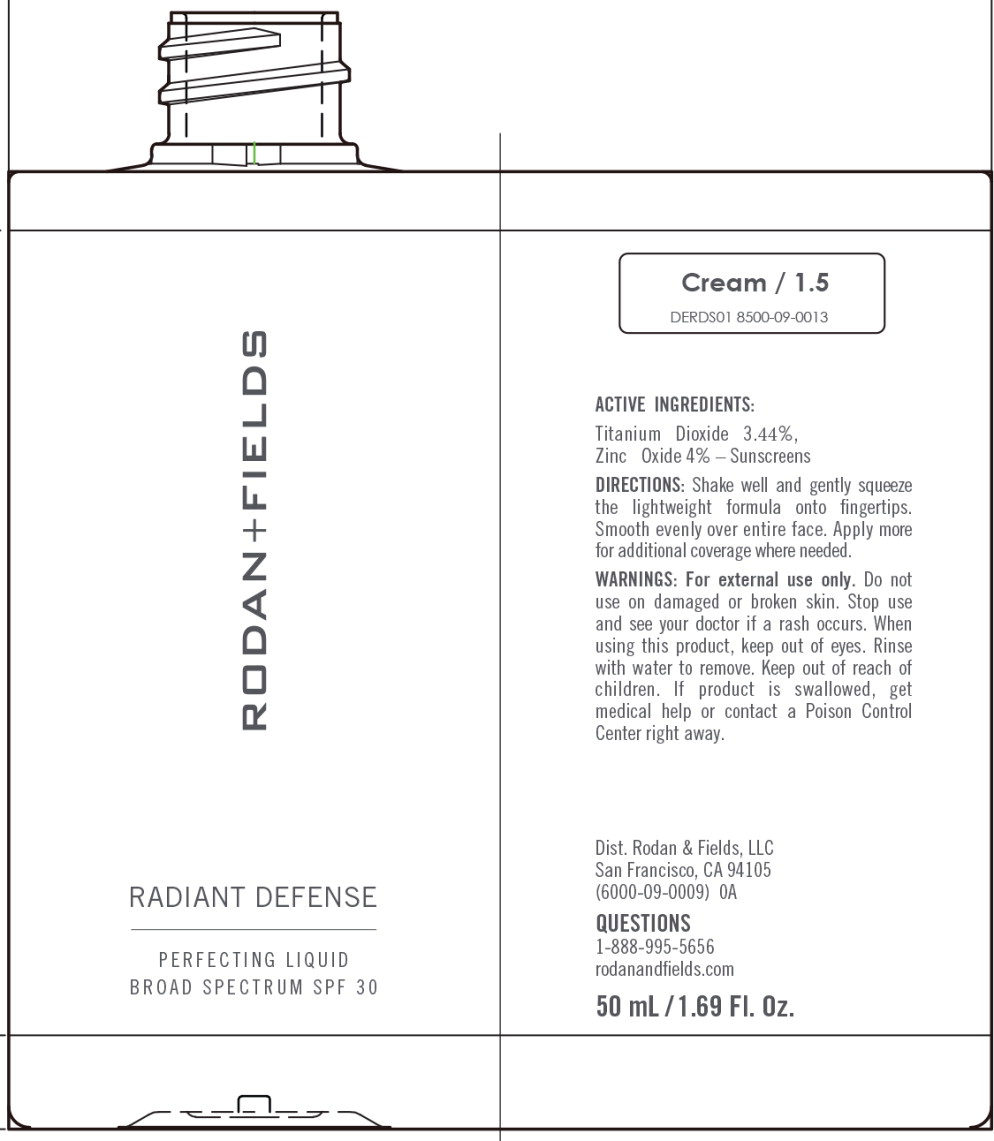
RADIANT DEFENSE PERFECTING LIQUID BROAD SPECTRUM SPF 30 - CREAM- titanium dioxide, zinc oxide lotion
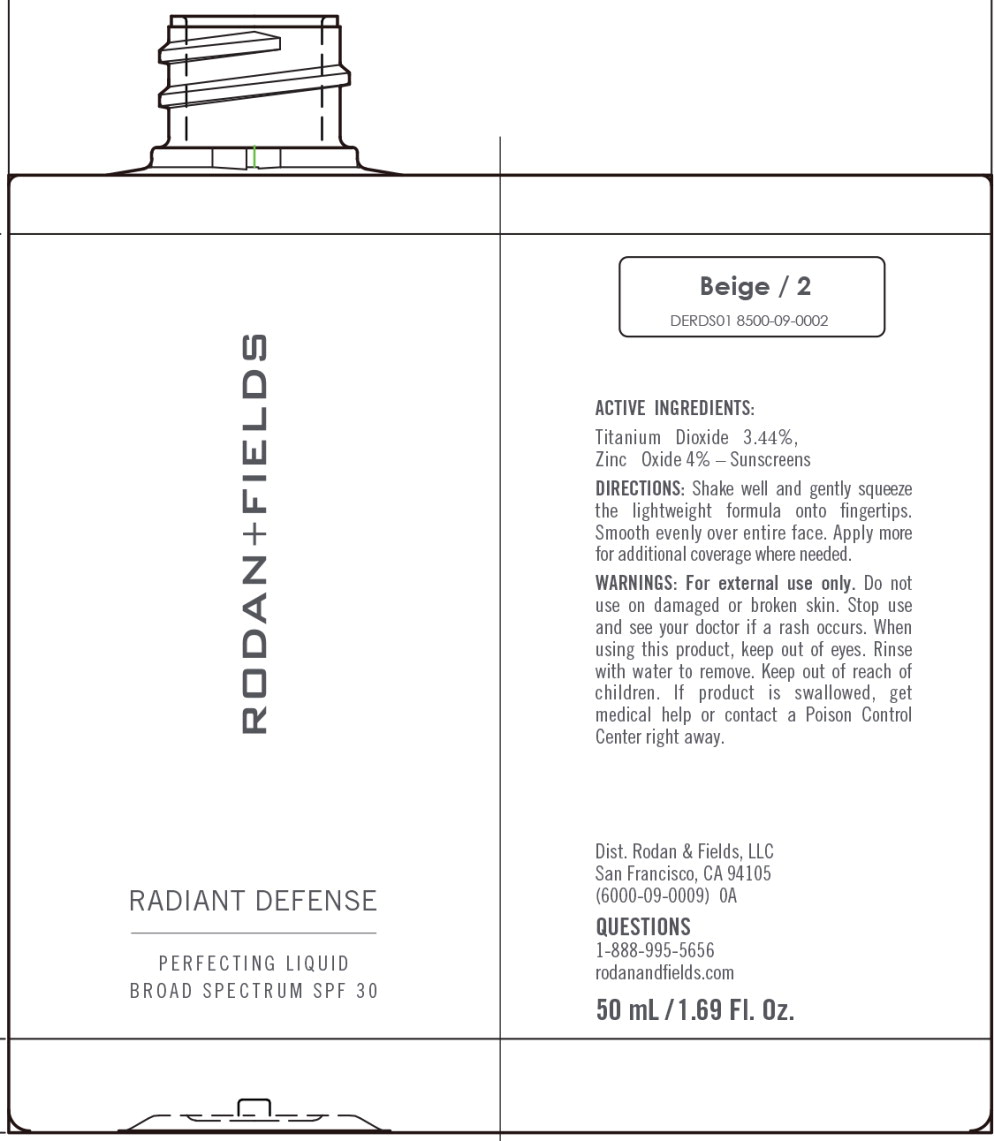
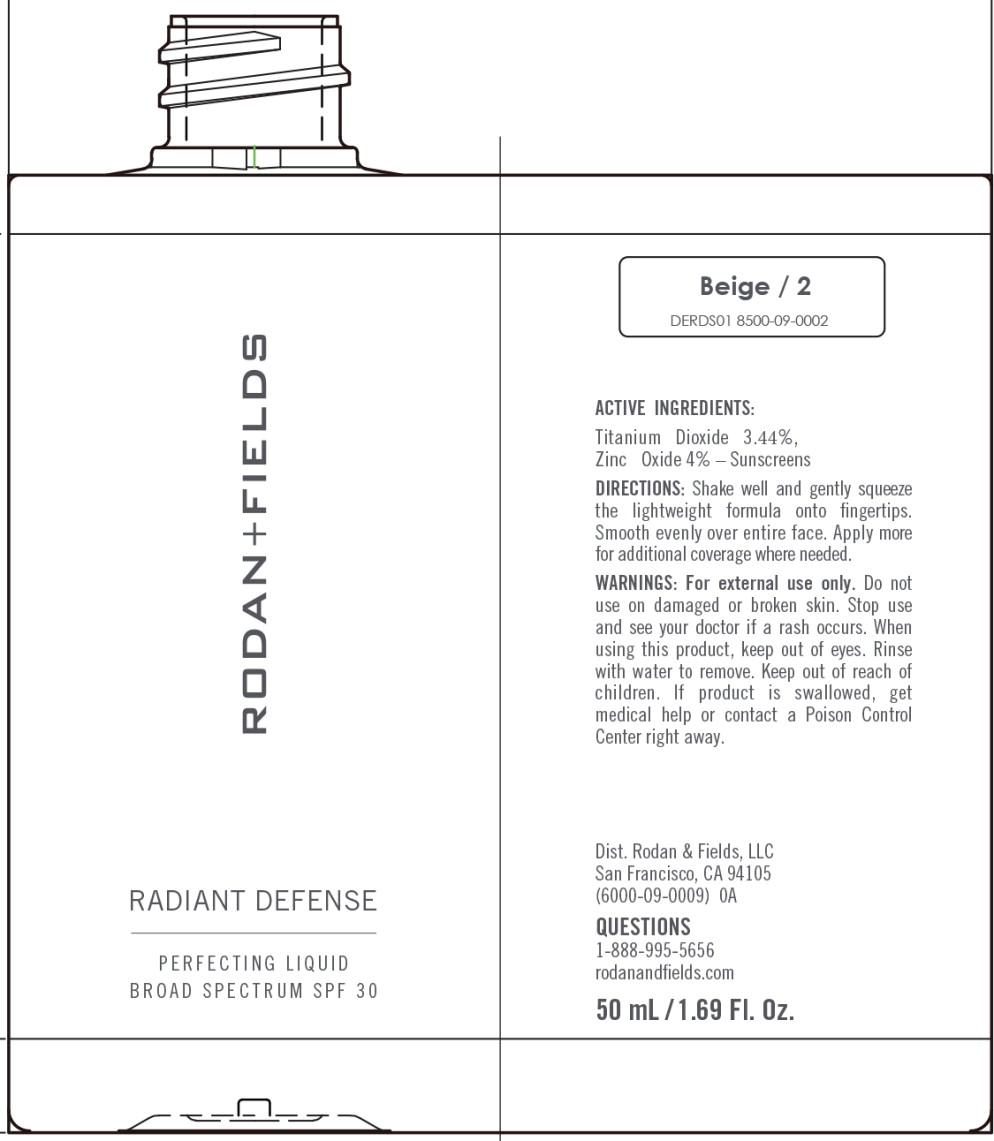
RADIANT DEFENSE PERFECTING LIQUID BROAD SPECTRUM SPF 30 - BEIGE- titanium dioxide, zinc oxide lotion
RADIANT DEFENSE PERFECTING LIQUID BROAD SPECTRUM SPF 30 - SAND- titanium dioxide, zinc oxide lotion
RADIANT DEFENSE PERFECTING LIQUID BROAD SPECTRUM SPF 30 - GOLDEN- titanium dioxide, zinc oxide lotion
RADIANT DEFENSE PERFECTING LIQUID BROAD SPECTRUM SPF 30 - HONEY- titanium dioxide, zinc oxide lotion
RADIANT DEFENSE PERFECTING LIQUID BROAD SPECTRUM SPF 30 - ALMOND- titanium dioxide, zinc oxide lotion
RADIANT DEFENSE PERFECTING LIQUID BROAD SPECTRUM SPF 30 - CARAMEL- titanium dioxide, zinc oxide lotion
RADIANT DEFENSE PERFECTING LIQUID BROAD SPECTRUM SPF 30 - ESPRESSO- titanium dioxide, zinc oxide lotion
RADIANT DEFENSE PERFECTING LIQUID BROAD SPECTRUM SPF 30 - TRUFFLE- titanium dioxide, zinc oxide lotion
-
NDC Code(s):
14222-2030-1,
14222-2031-1,
14222-2032-1,
14222-2033-1, view more14222-2034-1, 14222-2035-1, 14222-2036-1, 14222-2037-1, 14222-2038-1, 14222-2039-1
- Packager: Rodan & Fields
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated December 13, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active Ingredients
- Purpose
-
Uses
- Helps prevent sunburn.
- If used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun.
- Warnings
-
Directions
- Shake well and gently squeeze the lightweight formula onto fingertips.
- Apply liberally 15 minutes before sun exposure.
- Reapply at least every 2 hours.
- Use a water-resistant sunscreen if swimming or sweating.
-
Sun Protection Measures.
Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF of 15 or higher and other sun protection measures including:- Limit time in the sun, especially from 10 a.m.–2 p.m.
- Wear long-sleeve shirts, pants, hats and sunglasses.
- Children under 6 months: Ask a doctor.
- Other Information
-
Inactive Ingredients
Water, Dimethicone, Cyclopentasiloxane, Phenyl Trimethicone, Butylene Glycol, Coco-Caprylate/Caprate, C12-15 Alkyl Benzoate, Caprylic/Capric Triglyceride, Cetyl PEG/PPG-10/1 Dimethicone, Polymethylsilsesquioxane, HDI/Trimethylol Hexyllactone Crosspolymer, Polysilicone-11, PEG/ PPG-18/18 Dimethicone, Honey Extract, Oligopeptide-10, Tetrapeptide- 16, Boswellia Serrata Water, Hydrolyzed Rhodophyceae Extract, Oryza Sativa (Rice) Bran Extract, Vaccinium Angustifolium (Blueberry) Fruit Extract, Ectoin, Carnosine, Hydroxyacetophenone, Butyrospermum Parkii (Shea) Butter, Biosaccharide Gum-4, Alumina, Bis-Ethylhexyl Hydroxydimethoxy Benzylmalonate, Cyclohexasiloxane, Disteardimonium Hectorite, Isopropyl Titanium Triisostearate, Isostearic Acid, Lecithin, Methyldihydrojasmonate, Polyglyceryl-3 Polyricinoleate, Polyhydroxystearic Acid, Polysorbate 20, Propanediol, Silica, Sodium Chloride, Stearic Acid, BHT, 1,2-Hexanediol, Caprylyl Glycol, Sodium Benzoate, Phenoxyethanol. May Contain (+/-): Titanium Dioxide (CI 77891), Iron Oxides (CI 77491, CI 77492, CI 77499).
- Questions
- Principal Display Panel – 50 mL Cream Box Label
- Principal Display Panel – 50 mL Cream Bottle Label
- Principal Display Panel – 50 mL Shell Box Label
- Principal Display Panel – 50 mL Shell Bottle Label
- Principal Display Panel – 50 mL Beige Box Label
- Principal Display Panel – 50 mL Beige Bottle Label
- Principal Display Panel – 50 mL Sand Box Label
- Principal Display Panel – 50 mL Sand Bottle Label
- Principal Display Panel – 50 mL Honey Box Label
- Principal Display Panel – 50 mL Honey Bottle Label
- Principal Display Panel – 50 mL Golden Box Label
- Principal Display Panel – 50 mL Golden Bottle Label
- Principal Display Panel – 50 mL Almond Box Label
- Principal Display Panel – 50 mL Golden Bottle Label
- Principal Display Panel – 50 mL Caramel Box Label
- Principal Display Panel – 50 mL Caramel Label
- Principal Display Panel – 50 mL Espresso Box Label
- Principal Display Panel – 50 mL Espresso Label
- Principal Display Panel – 50 mL Truffle Box Label
- Principal Display Panel – 50 mL Truffle Label
-
INGREDIENTS AND APPEARANCE
RADIANT DEFENSE PERFECTING LIQUID BROAD SPECTRUM SPF 30 - SHELL
titanium dioxide, zinc oxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:14222-2030 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 3.44 g in 100 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 4.0 g in 100 mL Inactive Ingredients Ingredient Name Strength 1,2-HEXANEDIOL (UNII: TR046Y3K1G) ALUMINUM OXIDE (UNII: LMI26O6933) BIOSACCHARIDE GUM-4 (UNII: 9XRL057X90) BIS-ETHYLHEXYL HYDROXYDIMETHOXY BENZYLMALONATE (UNII: 7D4Q5YJ8NV) INDIAN FRANKINCENSE (UNII: 4PW41QCO2M) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) SHEA BUTTER (UNII: K49155WL9Y) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CARNOSINE (UNII: 8HO6PVN24W) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 3) (UNII: G300307ZXP) COCO-CAPRYLATE/CAPRATE (UNII: 8D9H4QU99H) CYCLOMETHICONE 6 (UNII: XHK3U310BA) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) DIMETHICONE (UNII: 92RU3N3Y1O) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) ECTOINE (UNII: 7GXZ3858RY) HEXAMETHYLENE DIISOCYANATE/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER (UNII: WB5K9Y35Y9) HONEY (UNII: Y9H1V576FH) HYDROXYACETOPHENONE (UNII: G1L3HT4CMH) ISOPROPYL TITANIUM TRIISOSTEARATE (UNII: 949E3KBJ1I) ISOSTEARIC ACID (UNII: X33R8U0062) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) METHYL DIHYDROJASMONATE (SYNTHETIC) (UNII: 3GW44CIE3Y) OLIGOPEPTIDE-10 (UNII: Q46328TRNK) RICE BRAN (UNII: R60QEP13IC) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) PHENOXYETHANOL (UNII: HIE492ZZ3T) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) POLYMETHYLSILSESQUIOXANE (4.5 MICRONS) (UNII: 59Z907ZB69) POLYSORBATE 20 (UNII: 7T1F30V5YH) PROPANEDIOL (UNII: 5965N8W85T) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM BENZOATE (UNII: OJ245FE5EU) SODIUM CHLORIDE (UNII: 451W47IQ8X) STEARIC ACID (UNII: 4ELV7Z65AP) LOWBUSH BLUEBERRY (UNII: G90PX41VP0) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:14222-2030-1 1 in 1 CARTON 03/10/2020 1 50 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 03/10/2020 RADIANT DEFENSE PERFECTING LIQUID BROAD SPECTRUM SPF 30 - CREAM
titanium dioxide, zinc oxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:14222-2031 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 3.44 g in 100 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 4.0 g in 100 mL Inactive Ingredients Ingredient Name Strength 1,2-HEXANEDIOL (UNII: TR046Y3K1G) ALUMINUM OXIDE (UNII: LMI26O6933) BIOSACCHARIDE GUM-4 (UNII: 9XRL057X90) BIS-ETHYLHEXYL HYDROXYDIMETHOXY BENZYLMALONATE (UNII: 7D4Q5YJ8NV) INDIAN FRANKINCENSE (UNII: 4PW41QCO2M) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) SHEA BUTTER (UNII: K49155WL9Y) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CARNOSINE (UNII: 8HO6PVN24W) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 3) (UNII: G300307ZXP) COCO-CAPRYLATE/CAPRATE (UNII: 8D9H4QU99H) CYCLOMETHICONE 6 (UNII: XHK3U310BA) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) DIMETHICONE (UNII: 92RU3N3Y1O) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) ECTOINE (UNII: 7GXZ3858RY) HEXAMETHYLENE DIISOCYANATE/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER (UNII: WB5K9Y35Y9) HONEY (UNII: Y9H1V576FH) HYDROXYACETOPHENONE (UNII: G1L3HT4CMH) ISOPROPYL TITANIUM TRIISOSTEARATE (UNII: 949E3KBJ1I) ISOSTEARIC ACID (UNII: X33R8U0062) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) METHYL DIHYDROJASMONATE (SYNTHETIC) (UNII: 3GW44CIE3Y) OLIGOPEPTIDE-10 (UNII: Q46328TRNK) RICE BRAN (UNII: R60QEP13IC) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) PHENOXYETHANOL (UNII: HIE492ZZ3T) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) POLYMETHYLSILSESQUIOXANE (4.5 MICRONS) (UNII: 59Z907ZB69) POLYSORBATE 20 (UNII: 7T1F30V5YH) PROPANEDIOL (UNII: 5965N8W85T) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM BENZOATE (UNII: OJ245FE5EU) SODIUM CHLORIDE (UNII: 451W47IQ8X) STEARIC ACID (UNII: 4ELV7Z65AP) LOWBUSH BLUEBERRY (UNII: G90PX41VP0) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:14222-2031-1 1 in 1 CARTON 03/10/2020 1 50 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 03/10/2020 RADIANT DEFENSE PERFECTING LIQUID BROAD SPECTRUM SPF 30 - BEIGE
titanium dioxide, zinc oxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:14222-2032 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 3.44 g in 100 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 4.0 g in 100 mL Inactive Ingredients Ingredient Name Strength 1,2-HEXANEDIOL (UNII: TR046Y3K1G) ALUMINUM OXIDE (UNII: LMI26O6933) BIOSACCHARIDE GUM-4 (UNII: 9XRL057X90) BIS-ETHYLHEXYL HYDROXYDIMETHOXY BENZYLMALONATE (UNII: 7D4Q5YJ8NV) INDIAN FRANKINCENSE (UNII: 4PW41QCO2M) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) SHEA BUTTER (UNII: K49155WL9Y) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CARNOSINE (UNII: 8HO6PVN24W) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 3) (UNII: G300307ZXP) COCO-CAPRYLATE/CAPRATE (UNII: 8D9H4QU99H) CYCLOMETHICONE 6 (UNII: XHK3U310BA) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) DIMETHICONE (UNII: 92RU3N3Y1O) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) ECTOINE (UNII: 7GXZ3858RY) HEXAMETHYLENE DIISOCYANATE/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER (UNII: WB5K9Y35Y9) HONEY (UNII: Y9H1V576FH) HYDROXYACETOPHENONE (UNII: G1L3HT4CMH) ISOPROPYL TITANIUM TRIISOSTEARATE (UNII: 949E3KBJ1I) ISOSTEARIC ACID (UNII: X33R8U0062) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) METHYL DIHYDROJASMONATE (SYNTHETIC) (UNII: 3GW44CIE3Y) OLIGOPEPTIDE-10 (UNII: Q46328TRNK) RICE BRAN (UNII: R60QEP13IC) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) PHENOXYETHANOL (UNII: HIE492ZZ3T) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) POLYMETHYLSILSESQUIOXANE (4.5 MICRONS) (UNII: 59Z907ZB69) POLYSORBATE 20 (UNII: 7T1F30V5YH) PROPANEDIOL (UNII: 5965N8W85T) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM BENZOATE (UNII: OJ245FE5EU) SODIUM CHLORIDE (UNII: 451W47IQ8X) STEARIC ACID (UNII: 4ELV7Z65AP) LOWBUSH BLUEBERRY (UNII: G90PX41VP0) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:14222-2032-1 1 in 1 CARTON 03/10/2020 1 50 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 03/10/2020 RADIANT DEFENSE PERFECTING LIQUID BROAD SPECTRUM SPF 30 - SAND
titanium dioxide, zinc oxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:14222-2033 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 3.44 g in 100 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 4.0 g in 100 mL Inactive Ingredients Ingredient Name Strength 1,2-HEXANEDIOL (UNII: TR046Y3K1G) ALUMINUM OXIDE (UNII: LMI26O6933) BIOSACCHARIDE GUM-4 (UNII: 9XRL057X90) BIS-ETHYLHEXYL HYDROXYDIMETHOXY BENZYLMALONATE (UNII: 7D4Q5YJ8NV) INDIAN FRANKINCENSE (UNII: 4PW41QCO2M) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) SHEA BUTTER (UNII: K49155WL9Y) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CARNOSINE (UNII: 8HO6PVN24W) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 3) (UNII: G300307ZXP) COCO-CAPRYLATE/CAPRATE (UNII: 8D9H4QU99H) CYCLOMETHICONE 6 (UNII: XHK3U310BA) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) DIMETHICONE (UNII: 92RU3N3Y1O) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) ECTOINE (UNII: 7GXZ3858RY) HEXAMETHYLENE DIISOCYANATE/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER (UNII: WB5K9Y35Y9) HONEY (UNII: Y9H1V576FH) HYDROXYACETOPHENONE (UNII: G1L3HT4CMH) ISOPROPYL TITANIUM TRIISOSTEARATE (UNII: 949E3KBJ1I) ISOSTEARIC ACID (UNII: X33R8U0062) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) METHYL DIHYDROJASMONATE (SYNTHETIC) (UNII: 3GW44CIE3Y) OLIGOPEPTIDE-10 (UNII: Q46328TRNK) RICE BRAN (UNII: R60QEP13IC) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) PHENOXYETHANOL (UNII: HIE492ZZ3T) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) POLYMETHYLSILSESQUIOXANE (4.5 MICRONS) (UNII: 59Z907ZB69) POLYSORBATE 20 (UNII: 7T1F30V5YH) PROPANEDIOL (UNII: 5965N8W85T) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM BENZOATE (UNII: OJ245FE5EU) SODIUM CHLORIDE (UNII: 451W47IQ8X) STEARIC ACID (UNII: 4ELV7Z65AP) LOWBUSH BLUEBERRY (UNII: G90PX41VP0) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:14222-2033-1 1 in 1 CARTON 03/10/2020 1 50 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 03/10/2020 RADIANT DEFENSE PERFECTING LIQUID BROAD SPECTRUM SPF 30 - GOLDEN
titanium dioxide, zinc oxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:14222-2034 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 3.44 g in 100 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 4.0 g in 100 mL Inactive Ingredients Ingredient Name Strength 1,2-HEXANEDIOL (UNII: TR046Y3K1G) ALUMINUM OXIDE (UNII: LMI26O6933) BIOSACCHARIDE GUM-4 (UNII: 9XRL057X90) BIS-ETHYLHEXYL HYDROXYDIMETHOXY BENZYLMALONATE (UNII: 7D4Q5YJ8NV) INDIAN FRANKINCENSE (UNII: 4PW41QCO2M) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) SHEA BUTTER (UNII: K49155WL9Y) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CARNOSINE (UNII: 8HO6PVN24W) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 3) (UNII: G300307ZXP) COCO-CAPRYLATE/CAPRATE (UNII: 8D9H4QU99H) CYCLOMETHICONE 6 (UNII: XHK3U310BA) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) DIMETHICONE (UNII: 92RU3N3Y1O) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) ECTOINE (UNII: 7GXZ3858RY) HEXAMETHYLENE DIISOCYANATE/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER (UNII: WB5K9Y35Y9) HONEY (UNII: Y9H1V576FH) HYDROXYACETOPHENONE (UNII: G1L3HT4CMH) ISOPROPYL TITANIUM TRIISOSTEARATE (UNII: 949E3KBJ1I) ISOSTEARIC ACID (UNII: X33R8U0062) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) METHYL DIHYDROJASMONATE (SYNTHETIC) (UNII: 3GW44CIE3Y) OLIGOPEPTIDE-10 (UNII: Q46328TRNK) RICE BRAN (UNII: R60QEP13IC) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) PHENOXYETHANOL (UNII: HIE492ZZ3T) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) POLYMETHYLSILSESQUIOXANE (4.5 MICRONS) (UNII: 59Z907ZB69) POLYSORBATE 20 (UNII: 7T1F30V5YH) PROPANEDIOL (UNII: 5965N8W85T) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM BENZOATE (UNII: OJ245FE5EU) SODIUM CHLORIDE (UNII: 451W47IQ8X) STEARIC ACID (UNII: 4ELV7Z65AP) LOWBUSH BLUEBERRY (UNII: G90PX41VP0) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:14222-2034-1 1 in 1 CARTON 03/10/2020 1 50 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 03/10/2020 RADIANT DEFENSE PERFECTING LIQUID BROAD SPECTRUM SPF 30 - HONEY
titanium dioxide, zinc oxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:14222-2035 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 3.44 g in 100 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 4.0 g in 100 mL Inactive Ingredients Ingredient Name Strength 1,2-HEXANEDIOL (UNII: TR046Y3K1G) ALUMINUM OXIDE (UNII: LMI26O6933) BIOSACCHARIDE GUM-4 (UNII: 9XRL057X90) BIS-ETHYLHEXYL HYDROXYDIMETHOXY BENZYLMALONATE (UNII: 7D4Q5YJ8NV) INDIAN FRANKINCENSE (UNII: 4PW41QCO2M) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) SHEA BUTTER (UNII: K49155WL9Y) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CARNOSINE (UNII: 8HO6PVN24W) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 3) (UNII: G300307ZXP) COCO-CAPRYLATE/CAPRATE (UNII: 8D9H4QU99H) CYCLOMETHICONE 6 (UNII: XHK3U310BA) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) DIMETHICONE (UNII: 92RU3N3Y1O) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) ECTOINE (UNII: 7GXZ3858RY) HEXAMETHYLENE DIISOCYANATE/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER (UNII: WB5K9Y35Y9) HONEY (UNII: Y9H1V576FH) HYDROXYACETOPHENONE (UNII: G1L3HT4CMH) ISOPROPYL TITANIUM TRIISOSTEARATE (UNII: 949E3KBJ1I) ISOSTEARIC ACID (UNII: X33R8U0062) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) METHYL DIHYDROJASMONATE (SYNTHETIC) (UNII: 3GW44CIE3Y) OLIGOPEPTIDE-10 (UNII: Q46328TRNK) RICE BRAN (UNII: R60QEP13IC) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) PHENOXYETHANOL (UNII: HIE492ZZ3T) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) POLYMETHYLSILSESQUIOXANE (4.5 MICRONS) (UNII: 59Z907ZB69) POLYSORBATE 20 (UNII: 7T1F30V5YH) PROPANEDIOL (UNII: 5965N8W85T) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM BENZOATE (UNII: OJ245FE5EU) SODIUM CHLORIDE (UNII: 451W47IQ8X) STEARIC ACID (UNII: 4ELV7Z65AP) LOWBUSH BLUEBERRY (UNII: G90PX41VP0) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:14222-2035-1 1 in 1 CARTON 03/10/2020 1 50 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 03/10/2020 RADIANT DEFENSE PERFECTING LIQUID BROAD SPECTRUM SPF 30 - ALMOND
titanium dioxide, zinc oxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:14222-2036 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 3.44 g in 100 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 4.0 g in 100 mL Inactive Ingredients Ingredient Name Strength 1,2-HEXANEDIOL (UNII: TR046Y3K1G) ALUMINUM OXIDE (UNII: LMI26O6933) BIOSACCHARIDE GUM-4 (UNII: 9XRL057X90) BIS-ETHYLHEXYL HYDROXYDIMETHOXY BENZYLMALONATE (UNII: 7D4Q5YJ8NV) INDIAN FRANKINCENSE (UNII: 4PW41QCO2M) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) SHEA BUTTER (UNII: K49155WL9Y) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CARNOSINE (UNII: 8HO6PVN24W) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 3) (UNII: G300307ZXP) COCO-CAPRYLATE/CAPRATE (UNII: 8D9H4QU99H) CYCLOMETHICONE 6 (UNII: XHK3U310BA) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) DIMETHICONE (UNII: 92RU3N3Y1O) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) ECTOINE (UNII: 7GXZ3858RY) HEXAMETHYLENE DIISOCYANATE/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER (UNII: WB5K9Y35Y9) HONEY (UNII: Y9H1V576FH) HYDROXYACETOPHENONE (UNII: G1L3HT4CMH) ISOPROPYL TITANIUM TRIISOSTEARATE (UNII: 949E3KBJ1I) ISOSTEARIC ACID (UNII: X33R8U0062) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) METHYL DIHYDROJASMONATE (SYNTHETIC) (UNII: 3GW44CIE3Y) OLIGOPEPTIDE-10 (UNII: Q46328TRNK) RICE BRAN (UNII: R60QEP13IC) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) PHENOXYETHANOL (UNII: HIE492ZZ3T) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) POLYMETHYLSILSESQUIOXANE (4.5 MICRONS) (UNII: 59Z907ZB69) POLYSORBATE 20 (UNII: 7T1F30V5YH) PROPANEDIOL (UNII: 5965N8W85T) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM BENZOATE (UNII: OJ245FE5EU) SODIUM CHLORIDE (UNII: 451W47IQ8X) STEARIC ACID (UNII: 4ELV7Z65AP) LOWBUSH BLUEBERRY (UNII: G90PX41VP0) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:14222-2036-1 1 in 1 CARTON 03/10/2020 1 50 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 03/10/2020 RADIANT DEFENSE PERFECTING LIQUID BROAD SPECTRUM SPF 30 - CARAMEL
titanium dioxide, zinc oxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:14222-2037 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 3.44 g in 100 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 4.0 g in 100 mL Inactive Ingredients Ingredient Name Strength 1,2-HEXANEDIOL (UNII: TR046Y3K1G) ALUMINUM OXIDE (UNII: LMI26O6933) BIOSACCHARIDE GUM-4 (UNII: 9XRL057X90) BIS-ETHYLHEXYL HYDROXYDIMETHOXY BENZYLMALONATE (UNII: 7D4Q5YJ8NV) INDIAN FRANKINCENSE (UNII: 4PW41QCO2M) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) SHEA BUTTER (UNII: K49155WL9Y) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CARNOSINE (UNII: 8HO6PVN24W) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 3) (UNII: G300307ZXP) COCO-CAPRYLATE/CAPRATE (UNII: 8D9H4QU99H) CYCLOMETHICONE 6 (UNII: XHK3U310BA) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) DIMETHICONE (UNII: 92RU3N3Y1O) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) ECTOINE (UNII: 7GXZ3858RY) HEXAMETHYLENE DIISOCYANATE/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER (UNII: WB5K9Y35Y9) HONEY (UNII: Y9H1V576FH) HYDROXYACETOPHENONE (UNII: G1L3HT4CMH) ISOPROPYL TITANIUM TRIISOSTEARATE (UNII: 949E3KBJ1I) ISOSTEARIC ACID (UNII: X33R8U0062) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) METHYL DIHYDROJASMONATE (SYNTHETIC) (UNII: 3GW44CIE3Y) OLIGOPEPTIDE-10 (UNII: Q46328TRNK) RICE BRAN (UNII: R60QEP13IC) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) PHENOXYETHANOL (UNII: HIE492ZZ3T) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) POLYMETHYLSILSESQUIOXANE (4.5 MICRONS) (UNII: 59Z907ZB69) POLYSORBATE 20 (UNII: 7T1F30V5YH) PROPANEDIOL (UNII: 5965N8W85T) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM BENZOATE (UNII: OJ245FE5EU) SODIUM CHLORIDE (UNII: 451W47IQ8X) STEARIC ACID (UNII: 4ELV7Z65AP) LOWBUSH BLUEBERRY (UNII: G90PX41VP0) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:14222-2037-1 1 in 1 CARTON 03/10/2020 1 50 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 03/10/2020 RADIANT DEFENSE PERFECTING LIQUID BROAD SPECTRUM SPF 30 - ESPRESSO
titanium dioxide, zinc oxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:14222-2038 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 3.44 g in 100 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 4.0 g in 100 mL Inactive Ingredients Ingredient Name Strength 1,2-HEXANEDIOL (UNII: TR046Y3K1G) ALUMINUM OXIDE (UNII: LMI26O6933) BIOSACCHARIDE GUM-4 (UNII: 9XRL057X90) BIS-ETHYLHEXYL HYDROXYDIMETHOXY BENZYLMALONATE (UNII: 7D4Q5YJ8NV) INDIAN FRANKINCENSE (UNII: 4PW41QCO2M) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) SHEA BUTTER (UNII: K49155WL9Y) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CARNOSINE (UNII: 8HO6PVN24W) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 3) (UNII: G300307ZXP) COCO-CAPRYLATE/CAPRATE (UNII: 8D9H4QU99H) CYCLOMETHICONE 6 (UNII: XHK3U310BA) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) DIMETHICONE (UNII: 92RU3N3Y1O) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) ECTOINE (UNII: 7GXZ3858RY) HEXAMETHYLENE DIISOCYANATE/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER (UNII: WB5K9Y35Y9) HONEY (UNII: Y9H1V576FH) HYDROXYACETOPHENONE (UNII: G1L3HT4CMH) ISOPROPYL TITANIUM TRIISOSTEARATE (UNII: 949E3KBJ1I) ISOSTEARIC ACID (UNII: X33R8U0062) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) METHYL DIHYDROJASMONATE (SYNTHETIC) (UNII: 3GW44CIE3Y) OLIGOPEPTIDE-10 (UNII: Q46328TRNK) RICE BRAN (UNII: R60QEP13IC) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) PHENOXYETHANOL (UNII: HIE492ZZ3T) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) POLYMETHYLSILSESQUIOXANE (4.5 MICRONS) (UNII: 59Z907ZB69) POLYSORBATE 20 (UNII: 7T1F30V5YH) PROPANEDIOL (UNII: 5965N8W85T) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM BENZOATE (UNII: OJ245FE5EU) SODIUM CHLORIDE (UNII: 451W47IQ8X) STEARIC ACID (UNII: 4ELV7Z65AP) LOWBUSH BLUEBERRY (UNII: G90PX41VP0) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:14222-2038-1 1 in 1 CARTON 03/10/2020 1 50 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 03/10/2020 RADIANT DEFENSE PERFECTING LIQUID BROAD SPECTRUM SPF 30 - TRUFFLE
titanium dioxide, zinc oxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:14222-2039 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 3.44 g in 100 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 4.0 g in 100 mL Inactive Ingredients Ingredient Name Strength 1,2-HEXANEDIOL (UNII: TR046Y3K1G) ALUMINUM OXIDE (UNII: LMI26O6933) BIOSACCHARIDE GUM-4 (UNII: 9XRL057X90) BIS-ETHYLHEXYL HYDROXYDIMETHOXY BENZYLMALONATE (UNII: 7D4Q5YJ8NV) INDIAN FRANKINCENSE (UNII: 4PW41QCO2M) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) SHEA BUTTER (UNII: K49155WL9Y) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CARNOSINE (UNII: 8HO6PVN24W) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 3) (UNII: G300307ZXP) COCO-CAPRYLATE/CAPRATE (UNII: 8D9H4QU99H) CYCLOMETHICONE 6 (UNII: XHK3U310BA) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) DIMETHICONE (UNII: 92RU3N3Y1O) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) ECTOINE (UNII: 7GXZ3858RY) HEXAMETHYLENE DIISOCYANATE/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER (UNII: WB5K9Y35Y9) HONEY (UNII: Y9H1V576FH) HYDROXYACETOPHENONE (UNII: G1L3HT4CMH) ISOPROPYL TITANIUM TRIISOSTEARATE (UNII: 949E3KBJ1I) ISOSTEARIC ACID (UNII: X33R8U0062) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) METHYL DIHYDROJASMONATE (SYNTHETIC) (UNII: 3GW44CIE3Y) OLIGOPEPTIDE-10 (UNII: Q46328TRNK) RICE BRAN (UNII: R60QEP13IC) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) PHENOXYETHANOL (UNII: HIE492ZZ3T) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) POLYMETHYLSILSESQUIOXANE (4.5 MICRONS) (UNII: 59Z907ZB69) POLYSORBATE 20 (UNII: 7T1F30V5YH) PROPANEDIOL (UNII: 5965N8W85T) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM BENZOATE (UNII: OJ245FE5EU) SODIUM CHLORIDE (UNII: 451W47IQ8X) STEARIC ACID (UNII: 4ELV7Z65AP) LOWBUSH BLUEBERRY (UNII: G90PX41VP0) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:14222-2039-1 1 in 1 CARTON 03/10/2020 1 50 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 03/10/2020 Labeler - Rodan & Fields (051659584)