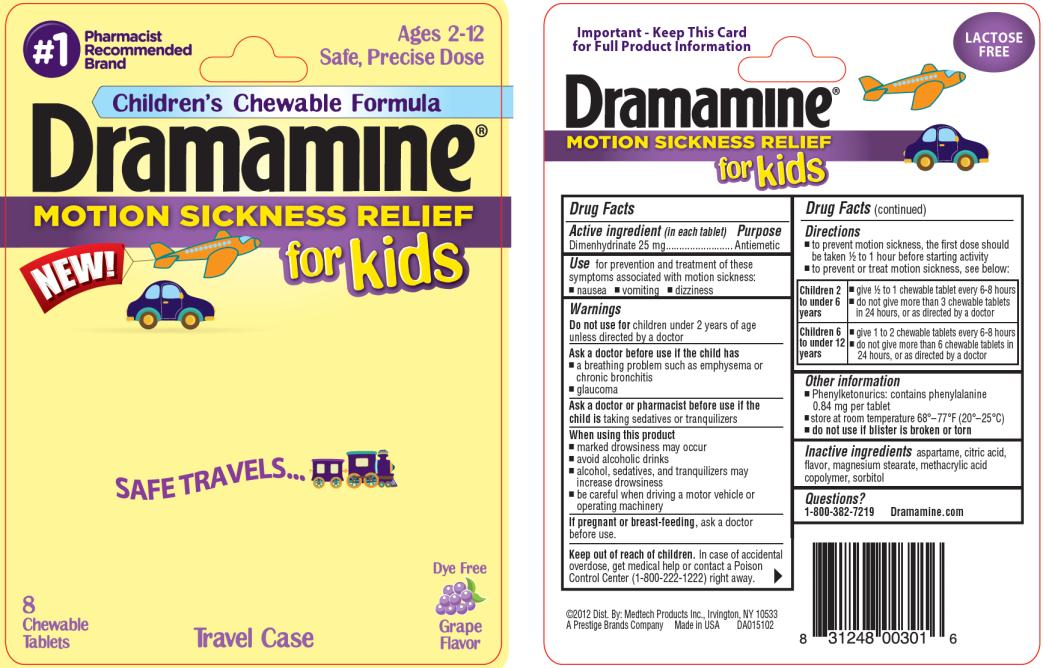
Label: DRAMAMINE FOR KIDS- dimenhydrinate tablet, chewable
-
Contains inactivated NDC Code(s)
NDC Code(s): 63029-904-01 - Packager: Medtech Products Inc.
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated April 13, 2012
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Use
-
Warnings
Ask a doctor before use if the child has
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- a breathing problem such as emphysema or chronic bronchitis
-
Directions
- to prevent motion sickness, the first dose should be taken ½ to 1 hour before starting activity
- to prevent or treat motion sickness, see below:
Children 2
to under 6
years- give ½ to 1 chewable tablet every 6-8 hours
- do not give more than 3 chewable tablets
in 24 hours, or as directed by a doctor
Children 6
to under 12
years- give 1 to 2 chewable tablets every 6-8 hours
- do not give more than 6 chewable tablets in
24 hours, or as directed by a doctor
- to prevent motion sickness, the first dose should be taken ½ to 1 hour before starting activity
- Other information
- Inactive ingredients
- Questions?
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
DRAMAMINE FOR KIDS
dimenhydrinate tablet, chewableProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:63029-904 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIMENHYDRINATE (UNII: JB937PER5C) (DIPHENHYDRAMINE - UNII:8GTS82S83M) DIMENHYDRINATE 25 mg Inactive Ingredients Ingredient Name Strength ASPARTAME (UNII: Z0H242BBR1) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) MAGNESIUM STEARATE (UNII: 70097M6I30) SORBITOL (UNII: 506T60A25R) Product Characteristics Color WHITE Score 2 pieces Shape ROUND Size 10mm Flavor GRAPE Imprint Code DRA Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63029-904-01 1 in 1 BLISTER PACK 1 8 in 1 CASE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part336 02/15/2012 Labeler - Medtech Products Inc. (122715688) Establishment Name Address ID/FEI Business Operations Contract Pharmacal Corporation 057795122 MANUFACTURE(63029-904)