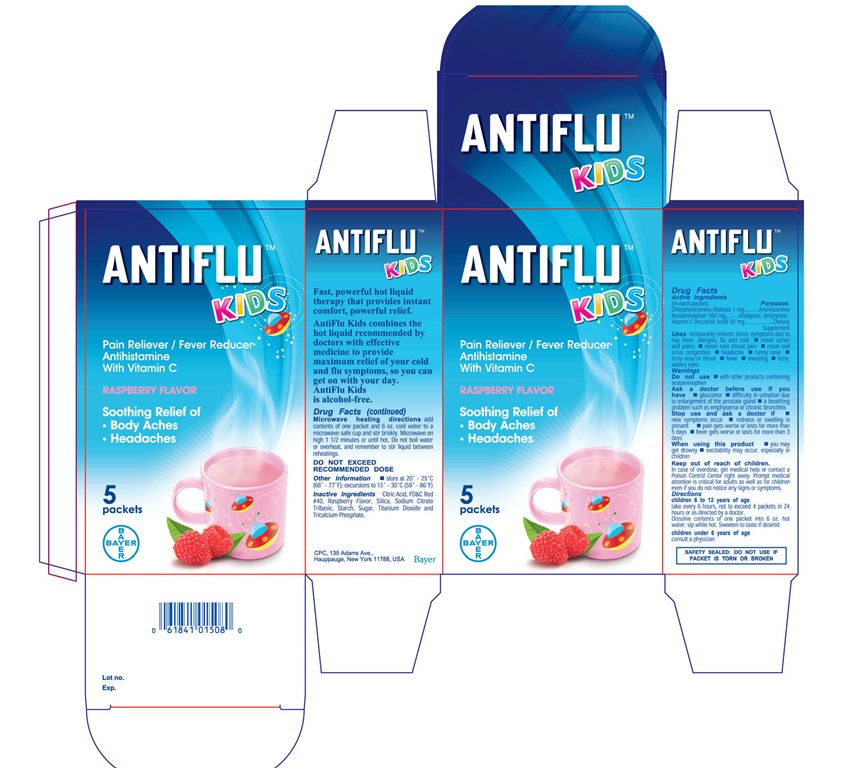
Label: ANTIFLU KIDS- chlorpl1eniramine maleate,acetaminophen powder, for solution
- NDC Code(s): 0280-0046-05
- Packager: Bayer HealthCare LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Export only
Drug Label Information
Updated December 13, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- ACTIVE INGREDIENT
- INDICATIONS & USAGE
- WARNINGS
- ASK DOCTOR
- ASK DOCTOR/PHARMACIST
- WHEN USING
- STOP USE
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- OTHER SAFETY INFORMATION
- INACTIVE INGREDIENT
- QUESTIONS
- PURPOSE
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ANTIFLU KIDS
chlorpl1eniramine maleate,acetaminophen powder, for solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0280-0046 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHLORPHENIRAMINE MALEATE (UNII: V1Q0O9OJ9Z) (CHLORPHENIRAMINE - UNII:3U6IO1965U) CHLORPHENIRAMINE MALEATE 1 mg in 13600 mg ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 160 mg in 13600 mg ASCORBIC ACID (UNII: PQ6CK8PD0R) (ASCORBIC ACID - UNII:PQ6CK8PD0R) ASCORBIC ACID 50 mg in 13600 mg Inactive Ingredients Ingredient Name Strength CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) FD&C RED NO. 40 (UNII: WZB9127XOA) Product Characteristics Color white Score Shape Size Flavor RASPBERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0280-0046-05 5 in 1 CARTON 04/02/2015 1 13.6 mg in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Export only 04/01/2015 Labeler - Bayer HealthCare LLC (112117283)