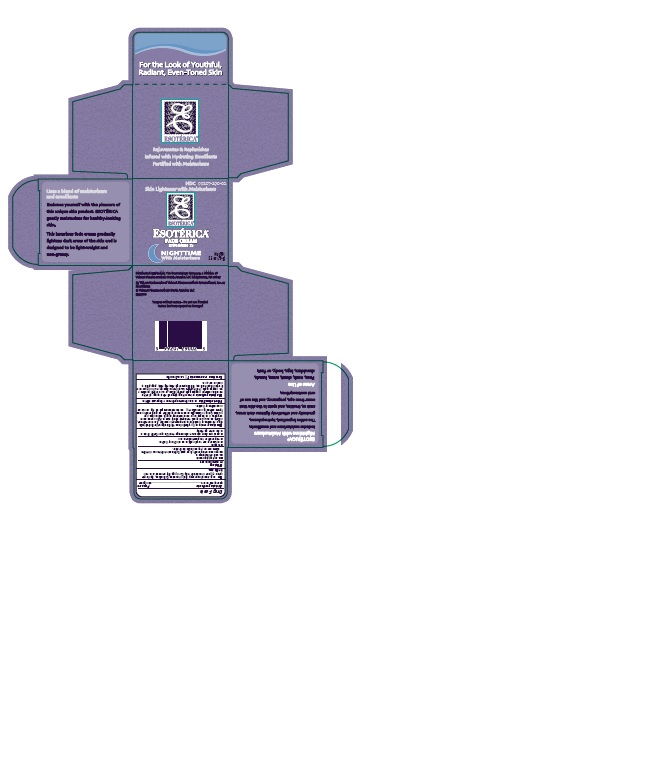
ESOTERICA NIGHTTIME WITH MOISTURIZERS- hydroquinone cream
Medicis Pharmaceutical Corp
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
ESOTÉRICA®
FADE CREAM
NIGHT TIME
With Moisturizers
Use
Lightens dark (brownish) areas on the skin such as freckles, age and liver spots, pigment in skin that may occur in pregnancy or from the use of oral contraceptives.
Warnings
For external use only.
Do not use
- •
- On inflamed or broken skin
- •
- On children under 12 years of age unless directed by a physician
Ask a doctor or pharmacist before use if you are taking products containing resorcinol, phenol, or salicylic acid
Directions
Adults
- •
- Apply a small amount as a thin layer to affected areas twice daily or as directed by a doctor
- •
- For sensitive skin, test overnight on small area (inside elbow)
- •
- If no improvement is seen after 3 months of treatment, discontinue use. On very dark skin, lightening effect may not be noticeable
- •
- Sun exposure should be limited by using a sunscreen agent, a sun blocking agent, or protective clothing to cover bleached skin when using and after using this product in order to prevent darkening from reoccurring.
Other information
- •
- Store at room temperature
- •
- Tamper-evident carton
- •
- Serious side effects may be reported to the phone number provided in the Questions? section below.
Inactive ingredients
purified water USP, glyceryl stearate, isopropyl palmitate NF, light mineral oil NF, ozokerite, propylene glycol USP, stearyl alcohol NF, PEG-1500 stearate, poloxamer 188, propylene glycol monostearate NF, steareth-20, laureth-23, sodium metabisulfite NF, sodium lauryl sulfate NF, anhydrous citric acid USP, fragrance, methylparaben NF, dimethicone, propylparaben NF, edetate disodium, butylated hydroxyanisole
| ESOTERICA
NIGHTTIME WITH MOISTURIZERS
hydroquinone cream |
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Medicis Pharmaceutical Corp (182837492) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Contract Pharmaceutical Limited | 248761249 | MANUFACTURE(99207-290) | |