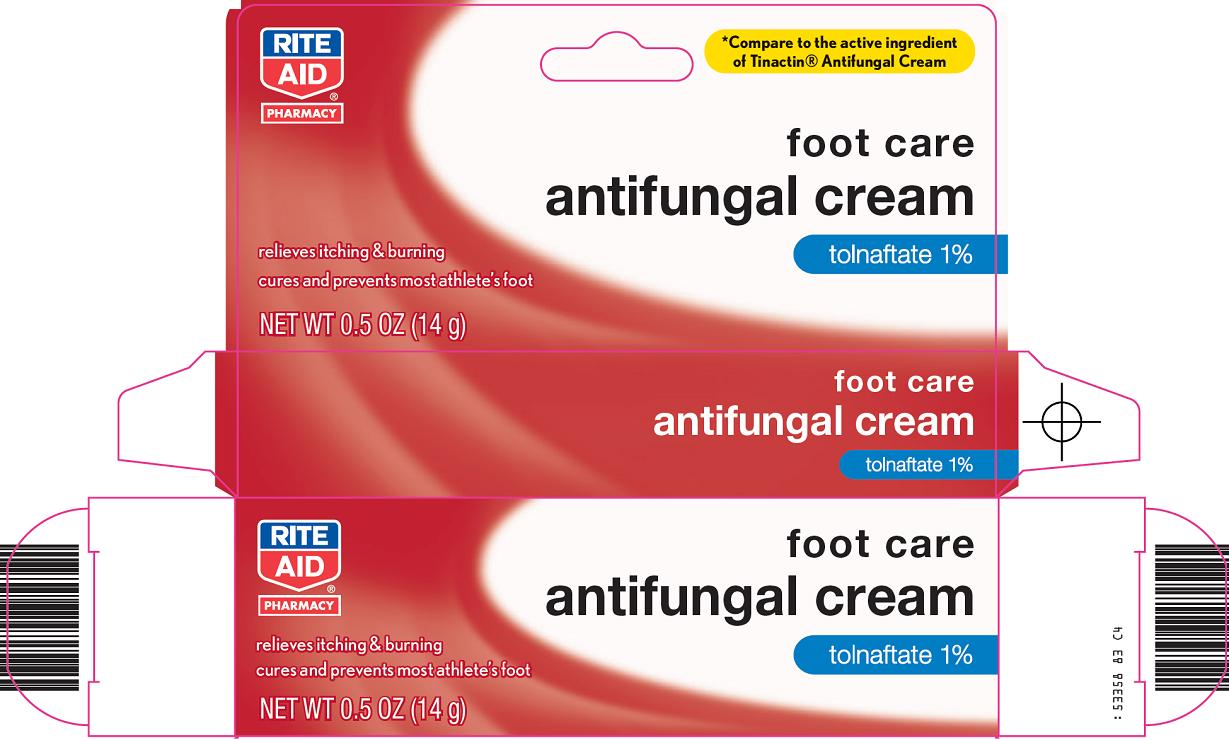
ANTIFUNGAL- tolnaftate cream
Rite Aid Corporation
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Rite Aid Corporation Antifungal Cream Drug Facts
Uses
- •
- proven clinically effective in the treatment of most
- •
- athlete’s foot (tinea pedis)
- •
- ringworm (tinea corporis)
- •
- helps prevent most athlete’s foot with daily use
- •
- for effective relief of
- •
- itching
- •
- burning
- •
- cracking
Directions
- •
- wash affected area and dry thoroughly
- •
- apply a thin layer over affected area twice daily (morning and night)
- •
- supervise children in the use of this product
- •
- for athlete’s foot: pay special attention to spaces between the toes. Wear well-fitting, ventilated shoes, and change shoes and socks at least once daily.
- •
- use daily for 4 weeks. If condition lasts longer, ask a doctor.
- •
- to prevent athlete’s foot, apply once or twice daily (morning and/or night)
- •
- this product is not effective on the scalp or nails
| ANTIFUNGAL
tolnaftate cream |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Rite Aid Corporation (014578892) |
Revised: 11/2017
Document Id: 488e7ea6-a2dc-4ed4-970a-2db87d3d0f0f
Set id: 129ec705-dab6-4e71-900a-1ee5d4945ce5
Version: 2
Effective Time: 20171107
Rite Aid Corporation