ACETIC ACID - acetic acid solution
Rising Pharmaceuticals, Inc.
----------
Acetic Acid Otic Solution USP, 2%
DESCRIPTION
Acetic Acid Otic Solution USP is a nonaqueous solution of glacial acetic acid, USP (2%), in a propylene glycol vehicle containing benzethonium chloride, USP (0.02%); propylene glycol diacetate, NF (3%) and sodium acetate, USP (0.015%). It may also contain citric acid, USP.
The molecular formula for acetic acid is CH3COOH, with a molecular weight of 60.05. The structural formula is:
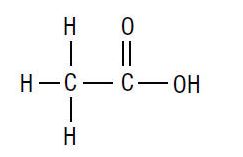
Acetic Acid Otic Solution is available as a nonaqueous otic solution buffered at pH 3 for use in the external ear canal.
CLINICAL PHARMACOLOGY
Acetic acid is anti-bacterial and anti-fungal; propylene glycol is hydrophilic and provides a low surface tension; benzethonium chloride is a surface active agent that promotes contact of the solution with tissues.
INDICATIONS AND USAGE
For the treatment of superficial infections of the external auditory canal caused by organisms susceptible to the action of the antimicrobial.
CONTRAINDICATIONS
Hypersensitivity to Acetic Acid Otic Solution or any of the ingredients. Perforated tympanic membrane is considered a contraindication to the use of any medication in the external ear canal.
PRECAUTIONS
Transient stinging or burning may be noted occasionally when the solution is first instilled into the acutely inflamed ear.
ADVERSE REACTIONS
Stinging or burning may be noted occasionally; local irritation has occurred very rarely.
DOSAGE AND ADMINISTRATION
Carefully remove all cerumen and debris to allow Acetic Acid Otic Solution to contact infected surfaces directly. To promote continuous contact, insert a wick of cotton saturated with the solution into the ear canal; the wick may also be saturated after insertion. Instruct the patient to keep the wick in for at least 24 hours and to keep it moist by adding 3 to 5 drops of the solution every 4 to 6 hours. The wick may be removed after 24 hours but the patient should continue to instill 5 drops of Acetic Acid Otic Solution 3 or 4 times daily thereafter, for as long as indicated. In pediatric patients, 3 to 4 drops may be sufficient due to the smaller capacity of the ear canal.
HOW SUPPLIED
Acetic Acid Otic Solution USP, 2% is available in 15 mL (NDC 64980-424-15) measured-drop, safety- tip plastic bottles.
STORAGE
Store at 20° to 25°C (68° to 77°F). [See USP Controlled Room Temperature.]
KEEP CONTAINER TIGHTLY CLOSED
Rx Only
Distributed by:
Rising Pharmaceuticals, Inc.
Saddle Brook, NJ 07663
Rev. 05/2018
MADE IN USA
Rev. 12/2018
| ACETIC ACID
acetic acid solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Rising Pharmaceuticals, Inc. (041241766) |

