LORATADINE ODT- loratadine tablet, orally disintegrating
Major Pharmaceuticals
----------
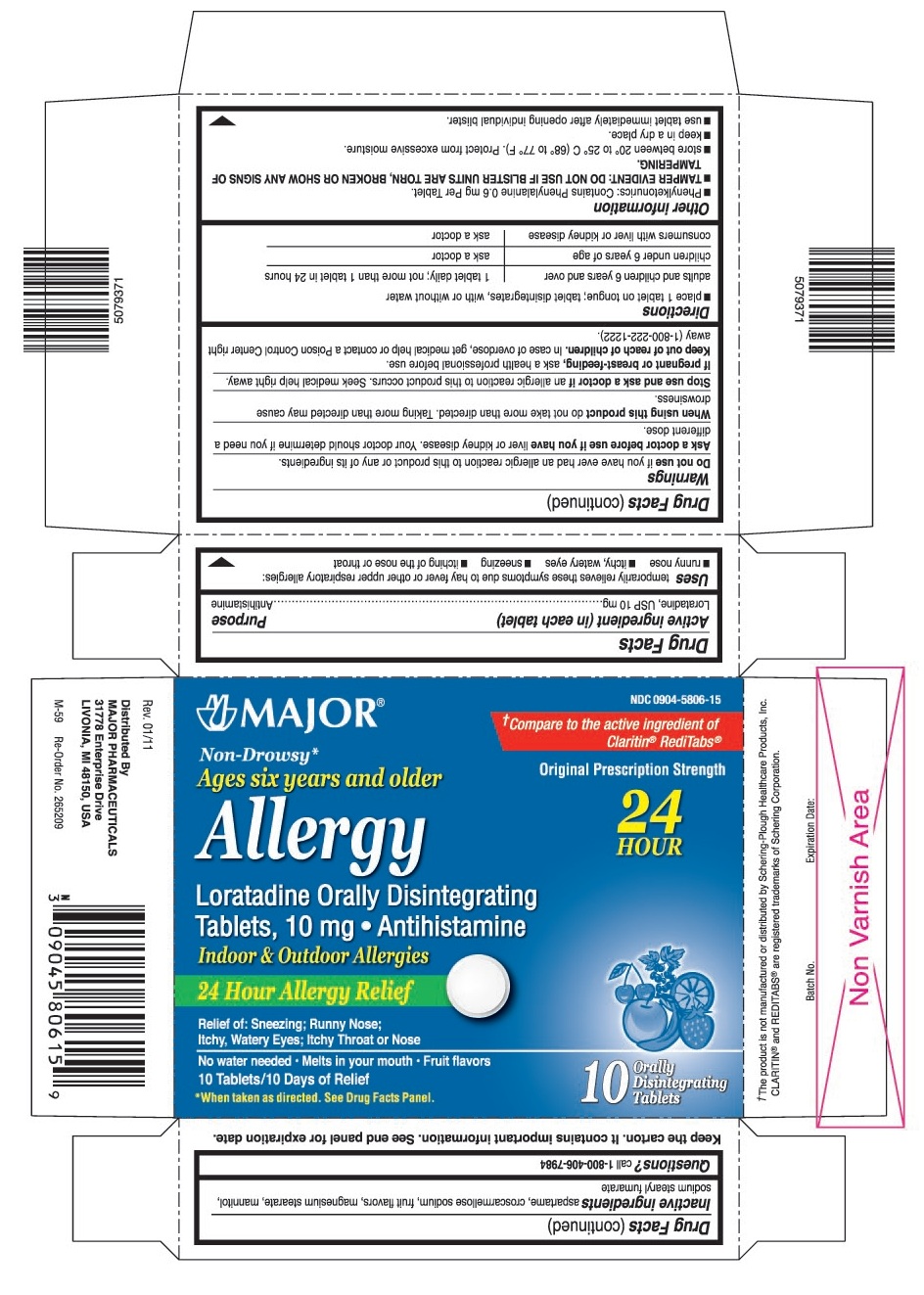
Drug Facts
USES
temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- •
- runny nose
- •
- itchy, watery eyes
- •
- sneezing
- •
- itching of the nose or throat
WARNINGS
Ask a doctor before use if you have
liver or kidney disease. Your doctor should determine if you need a different dose.
When using this product
do not take more than directed. Taking more than directed may cause drowsiness.
DIRECTIONS
- •
- place 1 tablet on tongue; tablet disintegrates, with or without water
|
adults and children 6 years and over |
1 tablet daily; not more than 1 tablet in 24 hours |
|
children under 6 years of age |
ask a doctor |
|
consumers with liver or kidney disease |
ask a doctor |
OTHER INFORMATION
- •
- Phenylketonurics: Contains Phenylalanine 0.6 mg Per Tablet.
- •
- TAMPER EVIDENT: DO NOT USE IF BLISTER UNITS ARE TORN, BROKEN OR SHOW ANY SIGNS OF TAMPERING.
- •
- store between 20° to 25° C (68° to 77° F). Protect from excessive moisture.
- •
- keep in a dry place.
- •
- use tablet immediately after opening individual blister.
INACTIVE INGREDIENTS
aspartame, croscarmellose sodium, fruit flavors, magnesium stearate, mannitol, sodium stearyl fumarate
QUESTIONS?
call 1-800-406-7984
Keep the carton. It contains important information.
See end panel for expiration date.
Distributed By
MAJOR PHARMACEUTICALS
31778 Enterprise Drive
LIVONIA, MI 48150, USA
PRINCIPAL DISPLAY PANEL
MAJOR®
NDC 0904-5806-15
Non-Drowsy*
Ages six years and older
Original Prescription Strength
Allergy
24 HOUR
Loratadine Orally Disintegrating Tablets, 10 mg
Antihistamine
Indoor & Outdoor Allergies
24 Hour Allergy Relief
Relief of:
- •
- Sneezing
- •
- Runny Nose
- •
- Itchy
- •
- Watery Eyes
- •
- Itchy Throat or Nose
No water needed.
Melts in your mouth.
Fruit Flavors
10 Tablets/10 Days of Relief
10 Orally Disintegrating Tablets
*When taken as directed. See Drug Facts Panel.
†Compare to the active ingredient of Claritin®RediTabs®
†This product is not manufactured or distributed by Schering-Plough Healthcare Products, Inc.
CLARITIN®and REDITABS®are registered trademarks of Schering Corporation.
| LORATADINE ODT
loratadine tablet, orally disintegrating |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Major Pharmaceuticals (191427277) |