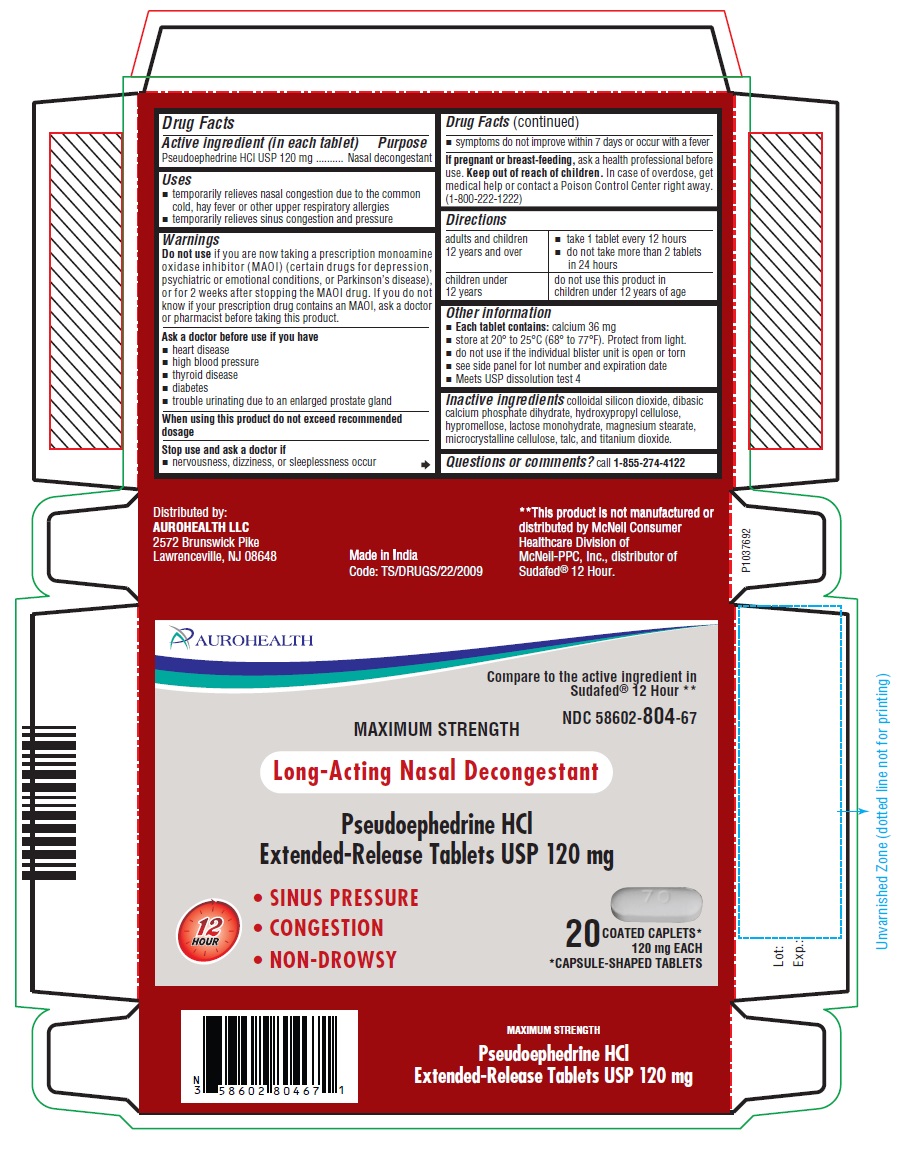
Label: PSEUDOEPHEDRINE HCL tablet, extended release
- NDC Code(s): 58602-804-67, 58602-804-83
- Packager: Aurohealth LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated November 3, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredient (in each tablet)
- Purpose
- Uses
- Warnings
-
Do not use
if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
- Ask a doctor before use if you have
- When using this product do not exceed recommended dosage
- Stop use and ask a doctor if
- If pregnant or breast-feeding,
- Keep out of reach of children.
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
- PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 120 mg, Blister Carton 20 (2 X 10) Extended-Release Tablets
-
INGREDIENTS AND APPEARANCE
PSEUDOEPHEDRINE HCL
pseudoephedrine hcl tablet, extended releaseProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58602-804 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PSEUDOEPHEDRINE HYDROCHLORIDE (UNII: 6V9V2RYJ8N) (PSEUDOEPHEDRINE - UNII:7CUC9DDI9F) PSEUDOEPHEDRINE HYDROCHLORIDE 120 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) DIBASIC CALCIUM PHOSPHATE DIHYDRATE (UNII: O7TSZ97GEP) HYDROXYPROPYL CELLULOSE (90000 WAMW) (UNII: UKE75GEA7F) HYPROMELLOSE 2208 (15000 MPA.S) (UNII: Z78RG6M2N2) HYPROMELLOSE 2208 (4000 MPA.S) (UNII: 39J80LT57T) HYPROMELLOSE 2910 (5 MPA.S) (UNII: R75537T0T4) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color WHITE (White to Off-white) Score no score Shape CAPSULE Size 18mm Flavor Imprint Code 70;T Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58602-804-83 1 in 1 CARTON 06/09/2017 1 10 in 1 BLISTER PACK; Type 0: Not a Combination Product 2 NDC:58602-804-67 2 in 1 CARTON 06/09/2017 2 10 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA209008 06/09/2017 Labeler - Aurohealth LLC (078728447) Establishment Name Address ID/FEI Business Operations Aurobindo Pharma Limited 650381903 ANALYSIS(58602-804) , MANUFACTURE(58602-804)