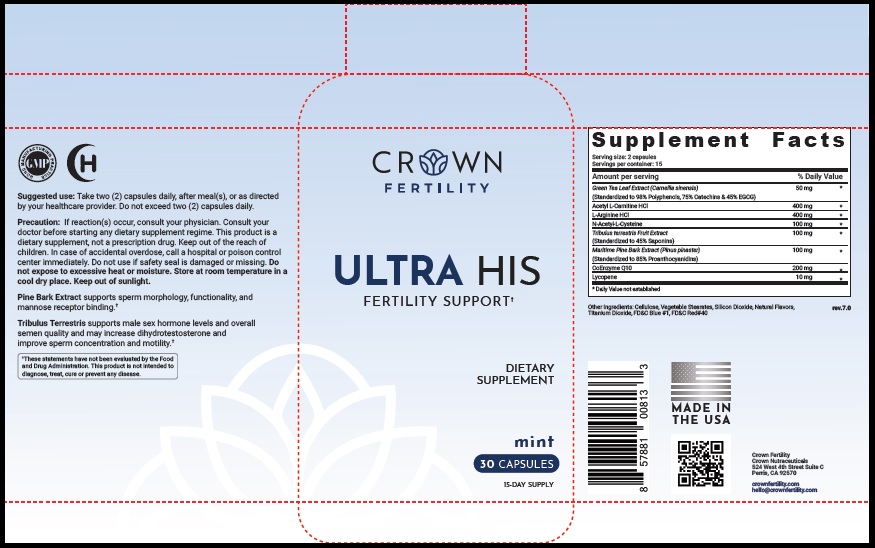
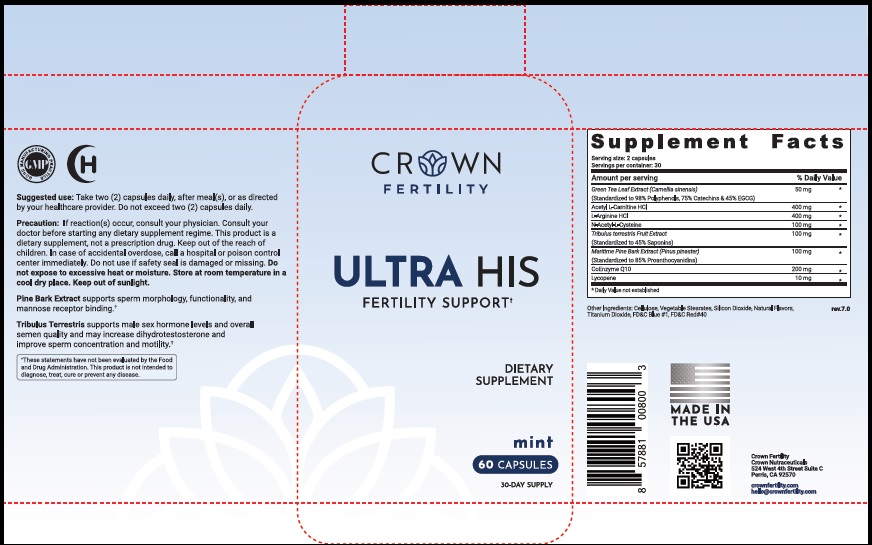
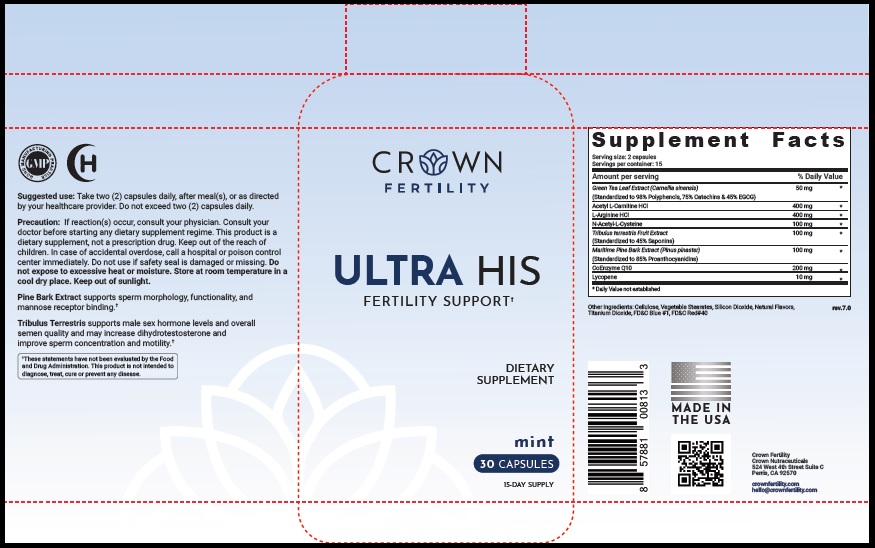
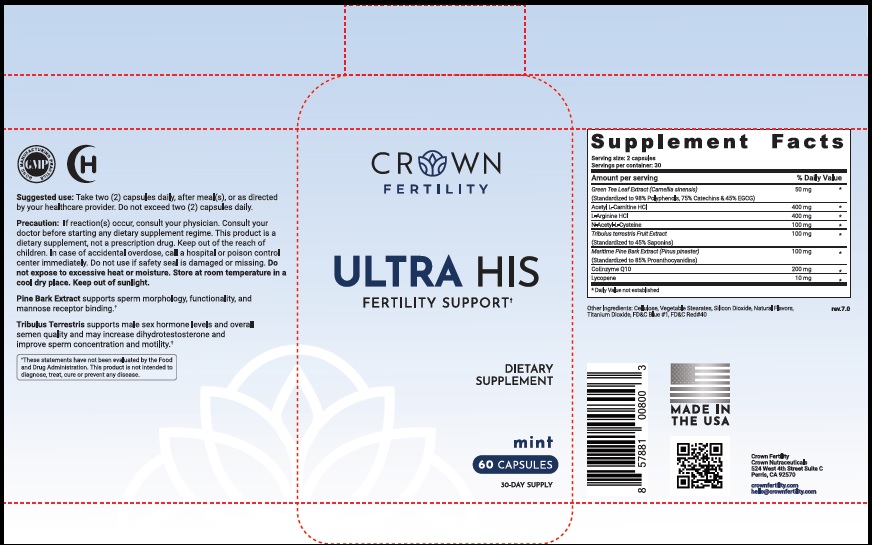
Label: ULTRA HIS MINT- green tea leaf, acetylcarnitine hydrochloride, arginine hydrochloride, n-monoacetylcystine, tribulus terrestris fruit, maritime pine, ubidecarenone , lycopene capsule
- NHRIC Code(s): 73489-005-01, 73489-005-02
- Packager: CROWN GENERAL AGENCY INC
- Category: DIETARY SUPPLEMENT
- DEA Schedule: None
- Marketing Status: Dietary Supplement
Drug Label Information
Updated October 22, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- STATEMENT OF IDENTITY
- Suggested Use
-
Precaution
If reaction(s) occur, consult your physician. Consult your doctor before starting any dietary supplement regime. This product is a dietary supplement, not a prescription drug. Keep out of the reach of children. In case of accidental overdose, call a hospital or poison control center immediately. Do not use if safety seal is damaged or missing. Do not expose to excessive heat or moisture. Store at room temperature in a cool dry place. Keep out of sunlight.
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ULTRA HIS MINT
green tea leaf, acetylcarnitine hydrochloride, arginine hydrochloride, n-monoacetylcystine, tribulus terrestris fruit, maritime pine, ubidecarenone , lycopene capsuleProduct Information Product Type DIETARY SUPPLEMENT Item Code (Source) NHRIC:73489-005 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GREEN TEA LEAF (UNII: W2ZU1RY8B0) (GREEN TEA LEAF - UNII:W2ZU1RY8B0) GREEN TEA LEAF 50 mg ACETYLCARNITINE HYDROCHLORIDE (UNII: NDW10MX58T) (ACETYLCARNITINE - UNII:6DH1W9VH8Q) ACETYLCARNITINE HYDROCHLORIDE 400 mg ARGININE HYDROCHLORIDE (UNII: F7LTH1E20Y) (ARGININE - UNII:94ZLA3W45F) ARGININE HYDROCHLORIDE 400 mg N-MONOACETYLCYSTINE (UNII: YXO5V2CF3F) (N-MONOACETYLCYSTINE - UNII:YXO5V2CF3F) N-MONOACETYLCYSTINE 100 mg TRIBULUS TERRESTRIS FRUIT (UNII: QNL076V6EQ) (TRIBULUS TERRESTRIS FRUIT - UNII:QNL076V6EQ) TRIBULUS TERRESTRIS FRUIT 100 mg MARITIME PINE (UNII: 50JZ5Z98QY) (TRIBULUS TERRESTRIS FRUIT - UNII:QNL076V6EQ) MARITIME PINE 100 mg UBIDECARENONE (UNII: EJ27X76M46) (UBIDECARENONE - UNII:EJ27X76M46) UBIDECARENONE 200 mg LYCOPENE (UNII: SB0N2N0WV6) (LYCOPENE - UNII:SB0N2N0WV6) LYCOPENE 10 mg Inactive Ingredients Ingredient Name Strength POWDERED CELLULOSE (UNII: SMD1X3XO9M) MAGNESIUM STEARATE (UNII: 70097M6I30) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C RED NO. 40 (UNII: WZB9127XOA) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:73489-005-01 30 in 1 BOTTLE, PLASTIC 2 NHRIC:73489-005-02 60 in 1 BOTTLE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date dietary supplement 11/29/2019 Supplement Facts Serving Size : Serving per Container : Amount Per Serving % Daily Value flavor color shape size (solid drugs) 19 mm scoring 1 Labeler - CROWN GENERAL AGENCY INC (067373836) Registrant - CROWN GENERAL AGENCY INC (067373836)