Label: CLEARCANAL EAR WAX SOFTERNER DROPS- carbamide peroxide solution/ drops
- NDC Code(s): 13709-230-01, 13709-230-10
- Packager: NeilMed Pharmaceuticals, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 11, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
-
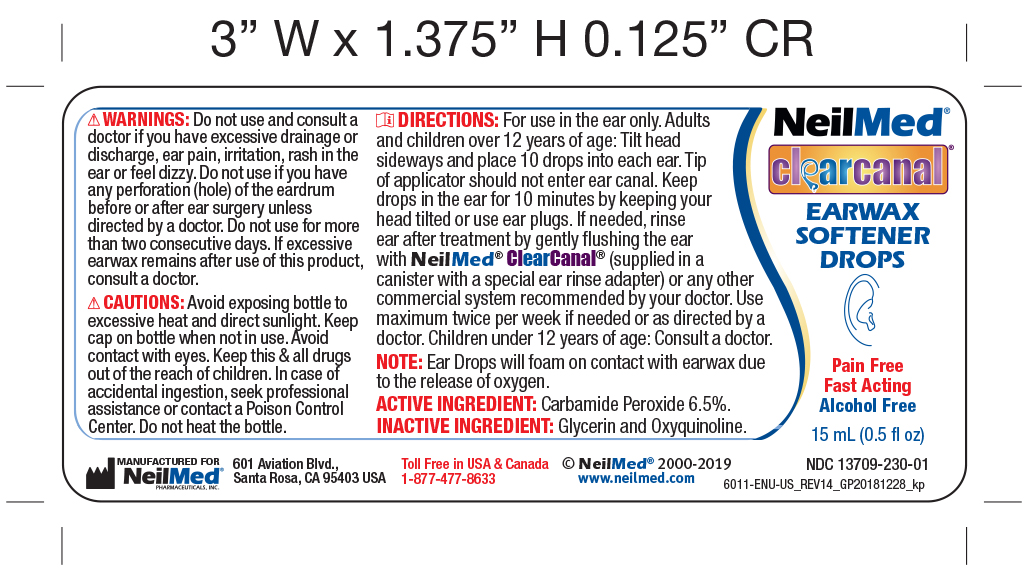
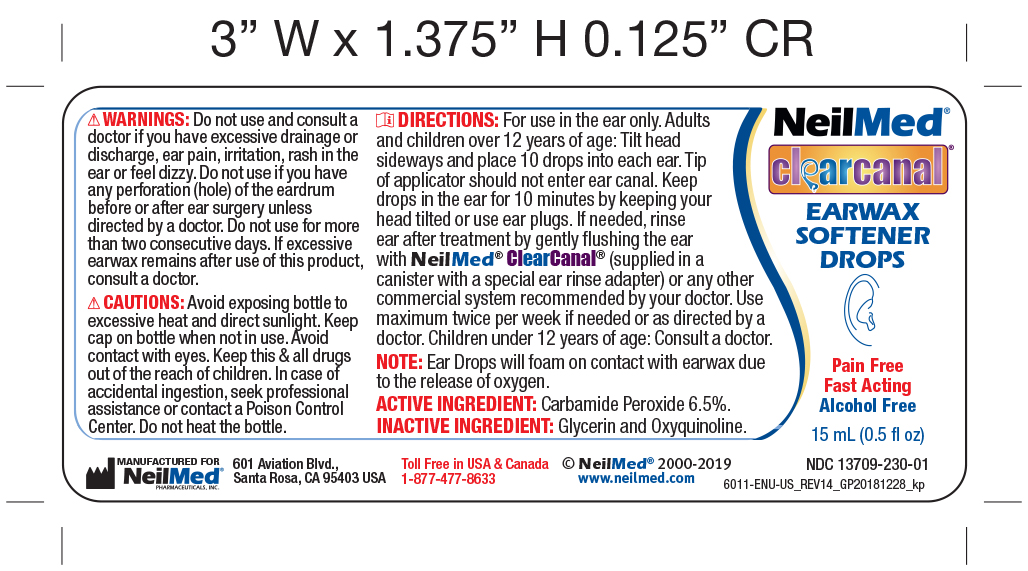
Ear Wax Removal Drops Directions
Step 1 Ear Wax Removal Drops Directions:
- FOR USE IN THE EAR ONLY.
- Adults and children over 12 years of age: tilt head sideways and place 5 to 10 drops into ear.
- Tip of applicator should not enter ear canal.
- Keep drops in ear for several minutes by keeping head tilted or placing enclosed ear plugs in ear.
- Use twice daily for upto 4 days if needed or as directed by a doctor.
- Any wax remaining after treatment may be removed by gently flushing the ear with NeilMed ClearCanal rinsing system with special ear rinse adapter.
- Children under 12 years of age: consult a doctor.
- STORAGE AND HANDLING
- INACTIVE INGREDIENT
- PURPOSE
- ASK DOCTOR
- KEEP OUT OF REACH OF CHILDREN
-
DO NOT USE
Do not use:
* If you have had an allergic reaction to Carbamide Peroxide.
* If you have ear drainage or discharge, ear pain, irritation or rash in the ear, or are dizzy; consult a doctor
* If you have an injury or perforation (hole) of the eardrum or after ear surgery, unless directed by a doctor
* For more than four consecutive days
- STOP USE
- WHEN USING
-
DESCRIPTION
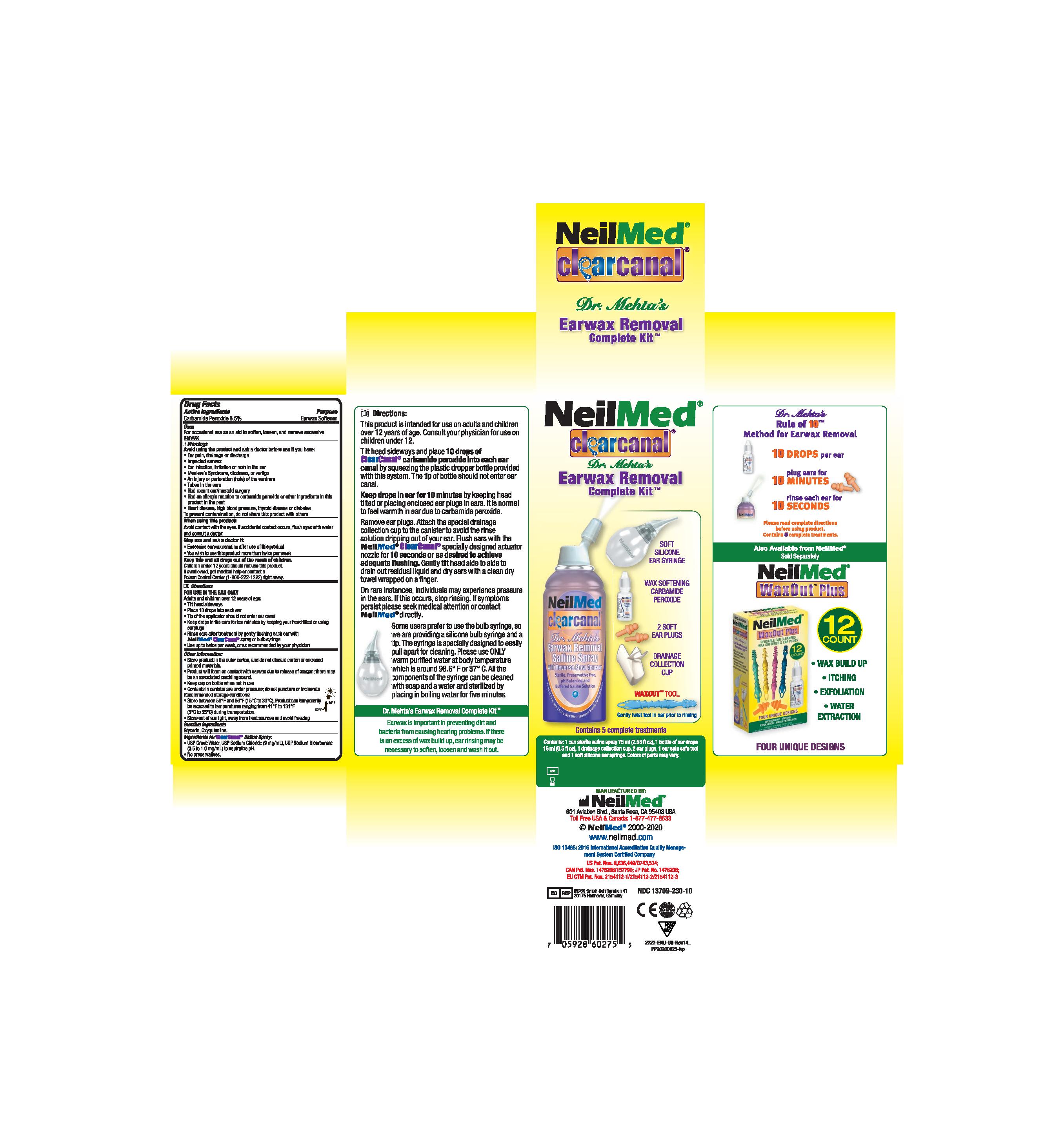
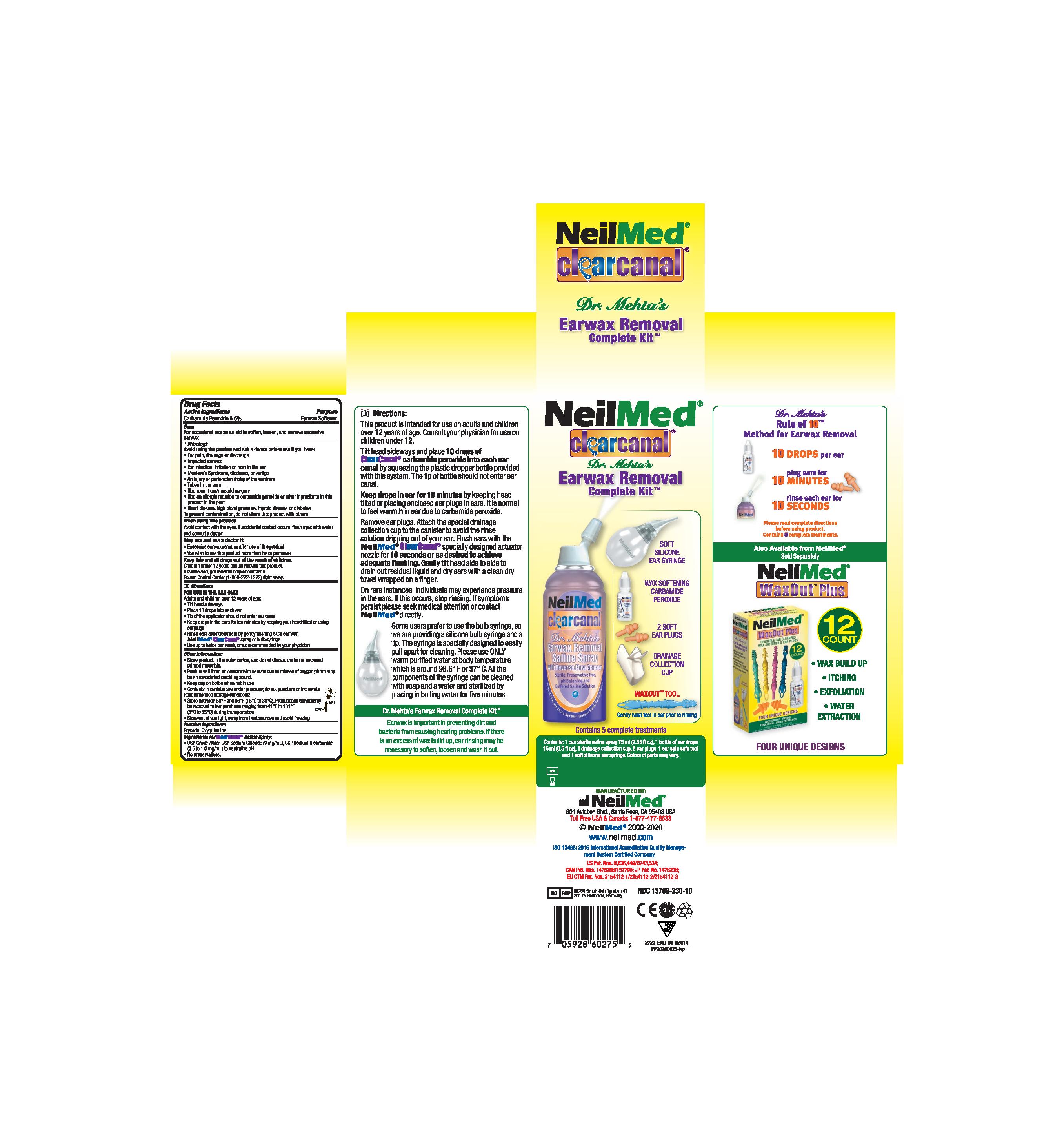
Ear Wax Removal Complete System
Ear wax is an important part of your body's system for keeping dirt, bacteria and other things from causing hearing problems. When rinsing is necessary, this cleaning system helps to soften, loosen and remove the excessive ear wax.
The system consists of kit with 1 bottle carbamide peroxide solution 15mL and 1 can sterile saline spray 177mL (or 125mL).
- DOSAGE & ADMINISTRATION
-
WARNINGS
Warnings:
Do not use:
If you have had an allergic reaction to carbamide peroxide.
If you have ear drainage or discharge, ear pain, irritation or rash in the ear, or are dizzy; consult a doctor
If you have an injury or perforation (hole) of the eardrum or after ear surgery, unless directed by a doctor
For more than four consecutive days
When using this product:
void contact with the eyes
Stop use ad ask a doctor if excessive ear wax remains after use of this product for four consecutive days. - PRINCIPAL DISPLAY PANEL
- ClearCanal Earwax Removal Kit_Product Label
-
INGREDIENTS AND APPEARANCE
CLEARCANAL EAR WAX SOFTERNER DROPS
carbamide peroxide solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13709-230 Route of Administration AURICULAR (OTIC) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CARBAMIDE PEROXIDE (UNII: 31PZ2VAU81) (HYDROGEN PEROXIDE - UNII:BBX060AN9V) CARBAMIDE PEROXIDE 65 mg in 1 mL Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) OXYQUINOLINE (UNII: 5UTX5635HP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13709-230-01 1 in 1 BOX 01/02/2012 1 15 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product 2 NDC:13709-230-10 1 in 1 KIT 08/24/2012 2 15 mL in 1 BOTTLE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M014 08/24/2011 Labeler - NeilMed Pharmaceuticals, Inc. (799295915) Establishment Name Address ID/FEI Business Operations NeilMed Pharmaceuticals, Inc. 799295915 manufacture(13709-230)